Drug-Induced Lymphadenopathy: Methotrexate
Definition
Lymphoproliferative disorders associated with methotrexate therapy, usually administered to patients with connective tissue diseases.
Introduction
Patients with immunodeficiency have an increased risk of lymphoproliferative disorders, in part, as a result of decreased host immunosurveillance (1). Well known examples of immunodeficiency associated with an increased frequency of lymphoproliferative disorders include patients with congenital immuno- deficiency syndromes or human immunodeficiency virus infection, and patients who have undergone solid organ or bone marrow transplantation. Patients with connective tissue diseases (and less commonly other autoimmune diseases), presumably as a result of immune dysfunction, also are known to have an increased risk of lymphoproliferative disorders (2). Complicating the analysis of lymphoma risk in these patients is the therapy they receive. A number of drugs have been implicated in increasing the risk of lymphoproliferative disorders, including methotrexate, azathioprine, sulfasalazine, steroids, antimalarial agents, and cytotoxic agents (e.g., cyclophosphamide).
Pathogenesis
The pathogenesis of lymphoproliferative disorders in patients with connective tissue diseases and other diseases (e.g., ulcerative colitis, psoriasis, etc.) receiving methotrexate therapy is poorly understood and is likely to be complex. These factors are likely related to the underlying disease (type, severity, and duration of inflammation) and the therapy used, including the intrinsic properties of the specific drug, dose, and duration of therapy. Patients often also receive multiple drugs. These factors are also intermingled, making it difficult to tease out the relative risk of each factor. It is difficult to determine how much of the risk of lymphoproliferative disorders can be attributed to chance alone versus severity of disease versus therapy. Although the answer to this conundrum is currently unknown, it appears clear that therapy is involved in pathogenesis because, in a subset of patients, discontinuing the drug results in regression or complete remission of the lymphoproliferative disorder (3,4). For this reason, others have suggested the term iatrogenic lymphoproliferative disorders as a designation for lymphoproliferative disorders that arise in this clinical setting (3).
Of the drugs implicated in the pathogenesis of lymphoproliferative disorders occurring in patients with connective tissue diseases, methotrexate is the agent most commonly involved. Methotrexate, formerly known as amethopterin, is a structural analog of folic acid that impairs DNA synthesis by inhibiting the enzyme dihydrofolate reductase. Methotrexate is used in the treatment of adult rheumatoid arthritis, severe psoriasis, dermatomyositis, post-transplantation reactions, and certain neoplastic diseases. For over 50 years, methotrexate has been shown to be effective in treating rheumatoid arthritis patients with severe symptoms (5). The mechanisms by which methotrexate ameliorates the symptoms of rheumatoid arthritis are poorly understood. Rheumatologists often prescribe methotrexate in small doses, in place of gold and azathioprine, because it is faster-acting and more effective (6,7,8). It has been estimated that the annual number of prescriptions
of methotrexate is 1.25 million; 50% to 60% of these are for patients with rheumatoid arthritis (9).
of methotrexate is 1.25 million; 50% to 60% of these are for patients with rheumatoid arthritis (9).
Although the frequency of methotrexate use in rheumatoid arthritis patients is a trivial explanation for this drug and this disease being most frequently associated with lymphoproliferative diseases, the sheer number of patients suggests that it is a factor. Patients with rheumatoid arthritis have impaired cell-mediated immunity and increased numbers of Epstein-Barr virus (EBV)-infected B cells in their blood (10,11,12). Methotrexate also has been shown to impair cell-mediated immune reactions to EBV-infected B cells, thus allowing for reactivation of latent infection (12,13). Nevertheless, the frequency of methotrexate-associated lymphoproliferative disorders in patients with rheumatoid arthritis is low. In one study of 16,263 rheumatoid arthritis patients at the Mayo Clinic, only 23 (1.4%) patients developed lymphoproliferative disorders, and only 6 (0.35%) patients had received methotrexate therapy (5).
Clinical Findings
The clinical features of patients with methotrexate-associated lymphoproliferative disorders can only be derived from small case series and the literature review of others. In 1996, Salloum et al. (13) summarized the findings in 37 patients, including nine new cases and 28 cases identified in the literature. Over 85% of patients had rheumatoid arthritis, with a small number of patients having dermatomyositis or rarely other diseases. The median age at time of diagnosis of lymphoproliferative disorder was 61 years (range, 15 to 86 years), with a female-to-male ratio of 2.2:1. The median time interval from initiation of methotrexate therapy until development of lymphoproliferative disorder was 3 years (range, 1.5 months to 12 years). Approximately 60% of patients received immunosuppressive drugs in addition to methotrexate, including corticosteroids, azathioprine, and cyclosporine. Approximately half the patients presented with lymphadenopathy, and the other half had extranodal sites of involvement. Lung and soft tissue were the most common extranodal sites. In the subset of 16 patients in whom methotrexate was discontinued and the patients were observed, six patients had spontaneous resolution of the lymphoproliferative disorder, and an additional four patients had partial resolution. In 1998, Sibilia et al. (14) reviewed the literature and identified 100 patients with rheumatoid arthritis treated with methotrexate who developed a lymphoproliferative disorder, including 48 patients with detailed information who had similar clinical and pathologic findings. In 14 patients treated by withdrawal of methotrexate alone, eight achieved spontaneous resolution (14). Kojima et al. (15) described 13 adults with rheumatoid arthritis (ages 52 to 78 years). These patients had rheumatoid arthritis for 1 to 35 years and had been treated with methotrexate 1 to 13 years prior to onset of a lymphoproliferative disorder. Seven of 13 patients presented with advanced-stage disease, and extranodal involvement was present in six patients. Hoshida et al. (16) have described 48 patients with rheumatoid arthritis who developed lymphoproliferative disorders while receiving methotrexate therapy. The median interval from initiation of methotrexate therapy to onset of lymphoproliferative disorder was 11 years. The pace of methotrexate-associated lymphoproliferative disorders in these studies was variable, either indolent (i.e., slow growth) or more aggressive. In both studies, a subset of patients underwent spontaneous resolution of their lymphoproliferative disorder by simply discontinuing methotrexate (15,16). In a recent editorial, Symmons estimated that 50 rheumatoid arthritis patients are reported in the literature who were treated with methotrexate, developed a lymphoproliferative disorder, and had the lesions regress following withdrawal of methotrexate (17).
Histologic Findings
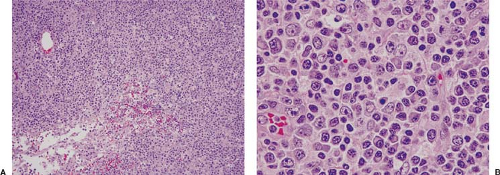
Lymphoproliferative disorders associated with methotrexate therapy are analogous to those that occur in other immunodeficiency states (3,4,5,6,13,14,15,16,18). Histologically, lesions can be polymorphous or monomorphous. Polymorphous lesions contain a variable mixture of small and larger lymphocytes, immunoblasts, histiocytes, and plasma cells (Fig. 45.1).
Reed-Sternberg-like cells can be present. The overall lymph node architecture is, in small or large part, preserved, and the findings fit within the spectrum of atypical lymphoid hyperplasia. Monomorphous lymphoproliferative disorders associated with methotrexate therapy more closely resemble diffuse large B-cell lymphoma, either the centroblastic or immunoblastic variant. Some of these lesions also show a spectrum, with both polymorphous and monomorphous areas (Fig. 45.2). In another subset of lymphoproliferative disorders associated with methotrexate therapy, the histologic findings closely resemble classical Hodgkin lymphoma with Reed-Sternberg or Hodgkin cells (or both) within a mixed cellular background including small lymphocytes, histiocytes, plasma cells, and granulocytes (Fig. 45.3).
Reed-Sternberg-like cells can be present. The overall lymph node architecture is, in small or large part, preserved, and the findings fit within the spectrum of atypical lymphoid hyperplasia. Monomorphous lymphoproliferative disorders associated with methotrexate therapy more closely resemble diffuse large B-cell lymphoma, either the centroblastic or immunoblastic variant. Some of these lesions also show a spectrum, with both polymorphous and monomorphous areas (Fig. 45.2). In another subset of lymphoproliferative disorders associated with methotrexate therapy, the histologic findings closely resemble classical Hodgkin lymphoma with Reed-Sternberg or Hodgkin cells (or both) within a mixed cellular background including small lymphocytes, histiocytes, plasma cells, and granulocytes (Fig. 45.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree