Chapter 14 Drug development
Introduction
Introduction
• Development accounts for about two-thirds of the total R&D costs. The cost per project is very much greater in the development phase, and increases sharply as the project moves into the later phases of clinical development. Keeping these costs under control is a major concern for management. Failure of a compound late in development represents a lot of money wasted.
• Speed in development is an important factor in determining sales revenue, as time spent in development detracts from the period of patent protection once the drug goes to market. As soon as the patent expires, generic competition sharply reduces sales revenue.
Despite a high level of awareness in the pharmaceutical industry of the need to reduce the money and time spent on development, both have actually increased significantly over the last two decades (see Chapter 22). This is mainly due to external factors, particularly the increased stringency applied by regulatory authorities in assessing the safety and efficacy of new compounds (see Chapter 20). The development burden is, therefore, tending to increase, thereby increasing the need for companies to improve their performance in this area in order to remain profitable and competitive.
The nature of drug development
• Technical development – solving technical problems relating to the synthesis and formulation of the drug substance, aimed mainly at ensuring the quality of the end-product:
• Investigative studies – establishing the safety and efficacy of the product, including assessment of whether it is pharmacokinetically suitable for clinical use in man:
 Coordination – managing quality control, logistics, communication and decision making in a large multidisciplinary project to ensure high-quality data and to avoid unnecessary delays:
Coordination – managing quality control, logistics, communication and decision making in a large multidisciplinary project to ensure high-quality data and to avoid unnecessary delays: Documentation and liaison with regulatory authorities – collating and presenting data of the type, quality and format needed to secure regulatory approval
Documentation and liaison with regulatory authorities – collating and presenting data of the type, quality and format needed to secure regulatory approvalBeyond Phase I, the route to be followed is generally much less well charted, and success depends to a much greater extent on strategic decisions by the project team as to which clinical indications should be investigated (see Chapter 17). They will need to assess, for example, whether recruiting patients to the trial will be easy or difficult, what exclusion criteria should apply, what clinical outcome measures should be used, and how long the treatment and assessment periods will need to be. To achieve registration as quickly as possible, it may, for example, be expedient to select a relatively low-market, but quick-to-test, clinical indication for the initial trials, and to run these trials in parallel with more prolonged trials in the major indication. Careful attention needs to be given to the patient group selected for the trial, so as to maximize the chance of success in obtaining a clear-cut result. Experience shows that inconclusive clinical trials resulting from poor decisions of this sort are a common cause of failure or delay in drug development. Where the indication allows this an adaptive trial design may allow a more efficient evaluation of the drug (see Chapter 17).
Components of drug development
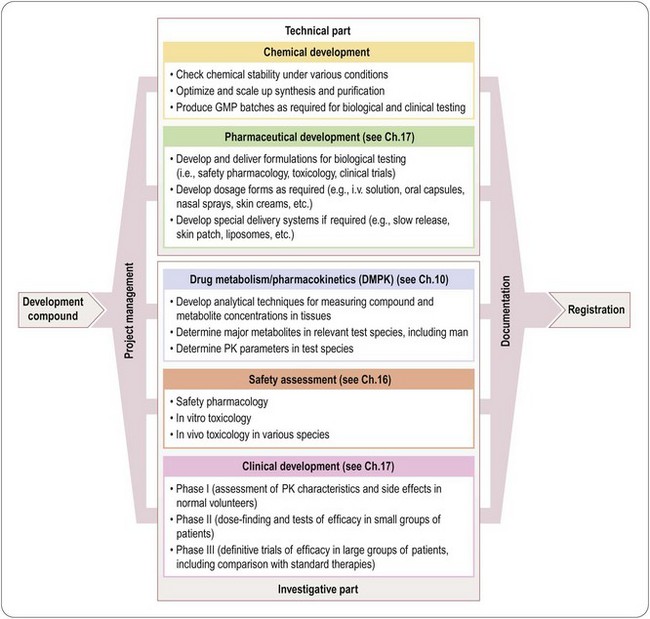
Figure 14.1 summarizes the main activities involved in developing a typical synthetic compound. It shows the main tasks that have to be completed before the compound can be submitted for regulatory approval, but needs to be translated into an operational plan (Figure 14.2) that will allow the project to proceed as quickly and efficiently as possible. It is obvious that certain tasks have to be completed in a particular order. For example, a supply of pure compound, prepared in an acceptable formulation, has to be available before Phase I clinical studies can begin. Animal toxicity data must also be available before the compound can be given to humans. Deciding on the dosage schedule to be used in efficacy trials requires knowledge of the pharmacokinetics and metabolism of the compound in humans. Because the data generated will be included in the final registration proposal, it is essential that each part of the work should be formally reported and ‘signed off’ by the group responsible and archived for future reference. A typical development project is likely to involve several hundred individuals, expert in different disciplines and working on different aspects of the project, and coordinating their work is a complex and demanding task. For this reason, most companies assign specialist project managers to this task. Their role is to design a project plan, based on input from the experts involved, to monitor progress and to adapt the plan accordingly. As well as being good organizers, project managers need to be excellent communicators, diplomatic, and with a good understanding of the scientific and technological aspects of the project. Figure 14.2 is a much-simplified outline of a project plan of the development of a typical orally active drug. Each ‘task’, represented by an arrow, starts and ends at a circular symbol (representing an ‘event’), and decision points are marked by diamond symbols. This type of graphical format, which is widely used as a project management tool and implemented in many commercially available software packages, is known as a PERT (project evaluation and review technique) chart. By assigning times – shortest possible, maximum, and expected – to each task, the timing of the whole project can be assessed and the critical path – i.e. the sequence of tasks that need to be completed on time in order to avoid an overall delay – defined. In Figure 14.2 the process has been reduced to a bare minimum to allow representation on a single page; in practice, each of the ‘tasks’ shown (e.g. develop formulations, perform Phase I studies, etc.) needs to be further subdivided into a series of subtasks and timings to enable the project to be planned and monitored at the operational level. The complete diagram for a typical drug development project will be of such size and complexity as to frighten all but the most hardened project management professionals. Software tools, fortunately, are available which allow the project to be viewed in different ways, such as Gantt charts, which are barcharts set against a calendar timescale, showing the expected start and completion dates for each task, many of which will be running simultaneously on any given date1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree