Dosing Antineoplastic Medications in Obese Patients
Brandon R. Shank
Outline
•Introduction
•Determining Body Surface Area
•Alternatives to Body Surface Area Dosing
•Specific Evidence by Cancer Type
•Antineoplastic Medications
•Conclusion
•Sample Calculation: Carboplatin
•Final Step: Always Use Clinical Judgment
•Summary Table: Antineoplastic Medication Dosing Recommendations in Obese Patients
Obesity causes about 20% of all cancers.1 It is known that adipose tissue secretes excessive amounts of estrogen, increasing patients’ risk for breast cancer, especially in postmenopausal women.2 A high-caloric diet and physical inactivity put individuals at an increased risk of colorectal cancer. Elevated body weight has been associated with an increase in mortality from the following cancers: esophagus, colon, rectal, liver, gallbladder, pancreas, kidney, non-Hodgkin lymphoma (NHL), and multiple myeloma.3 Challenges in nonpharmacologic interventions in obese patients include the inability to achieve negative margins during surgery, difficulty with optimal positioning of patients during radiation, and achieving deep enough radiation penetration to the tumor.2 This chapter will discuss disease-specific and antineoplastic medications data in relation to obese individuals.
In addition to the challenges seen with surgical and radiation management of obese patients with cancer, there are significant challenges with delivering adequate concentrations of antineoplastic medications to the tumor. Most antineoplastic drugs have a narrow therapeutic window so achieving the appropriate concentration is paramount. Many chemotherapy agents exhibit dose-dependent cytotoxic activity in vitro.4 High-dose therapy has been shown to decrease the emergence of resistant malignant clones.5 Delivering too small of a dose has translated into decreased efficacy in breast cancer patients and other types of cancer.6 However, administration of too large a dose has the potential to cause excessive toxicity. Consider the following when determining an antineoplastic drug dose:
Treatment intent—Be aggressive when treating patients for curative intent. Careful consideration should be made when dosing patients for palliation to maximize the patient’s quality of life so dose decreases may be warranted.
Weight assessment—Clinically evaluate patients and determine if their body composition is mostly muscle mass or body fat. For example, a body builder may appear overweight on paper but have a significant proportion of lean muscle mass in person. Excess fluid accumulation, such as that seen in ascites, should be assessed as certain drugs (such as methotrexate) can distribute into third-space fluids and cause greater toxicity. Most often total body weight (TBW) is used in the body surface area (BSA) calculation; however, some protocols use an adjusted body weight.
End-organ function—Many antineoplastic agents undergo hepatic or renal clearance. Hepatic flow may be decreased in patients with fatty liver disease, but some studies have shown increases in phase II conjugation reactions in this disease state.7 For renally cleared medications, adjustments in weight may need to be made to prevent overestimation of the patient’s creatinine clearance. See Chapter 1: Introduction to Dosing Medications in Obese Patients for a complete discussion of hepatic and renal changes in obese patients.
Performance status—Patients’ performance status can be used as a surrogate for their reserve and ability to tolerate treatment. Patients who are heavily pretreated or have significant comorbidities may not be able to tolerate as intensive treatment.
Determining Body Surface Area
Area under the curve (AUC) is a measure of drug exposure, which is directly related to response and toxicity for many antineoplastic medications. With the narrow therapeutic window of most antineoplastic agents, the goal in drug dosing is to achieve a certain AUC. One of the most significant challenges associated with obtaining the desired AUC is the difference in tumor penetration, due to chemical properties such as lipophilicity and protein binding, of specific agents.
An initial method to mimic AUC estimates is use of BSA that is most commonly used to dose antineoplastic medications. BSA was selected because it closely resembles cardiac output; thus, blood flow to the liver and kidneys where most medications are metabolized and eliminated. BSA allows scientists to easily extrapolate dosing from animals to humans. Some limitations to BSA dosing are that it does not account for body composition, end-organ function changes with age, impact of comorbidities, or sex-related differences.8
There are many different formulas to calculate BSA. Consensus opinion is that no formula performs better than another across a wide range of patients.9,10 Du Bois and Du Bois published the first BSA equation (Equation 6-1, Table 6-1) developed from nine individuals of various sizes, including one child and one obese patient (BMI 41.5 kg/m2).11 The Du Bois and Du Bois equation has been extensively validated. See Table 6-1 for a table of available BSA equations. About two decades later, Boyd devised a new, more complex formula from 1,114 individuals (Equation 6-2, Table 6-1).12 The Boyd formula was more accurate for determining BSA in infants and children.
Gehan and George worked to optimize the coefficients of the Du Bois and Du Bois equation, which was done by increasing the sample size (Equation 6-3, Table 6-1).13 The impact of age was tested by Gehan and George, but this did not enhance their equation’s accuracy. The largest patient included in the Gehan and George study was 98 kg. Gehan and George found that the Du Bois and Du Bois equation overestimates the BSA, whereas Haycock and colleagues (Equation 6-4, Table 6-1) determined the Du Bois and Du Bois equation underestimates results for those with a BSA of less than 0.7 m2 (newborn infants).14 The Haycock model included 81 individuals of a variety of ages (newborn to adults), weights (thin to obese), and ethnicities (Black, Hispanic, and White).
Table 6-1. Body Surface Area Equations with Strengths and Limitations
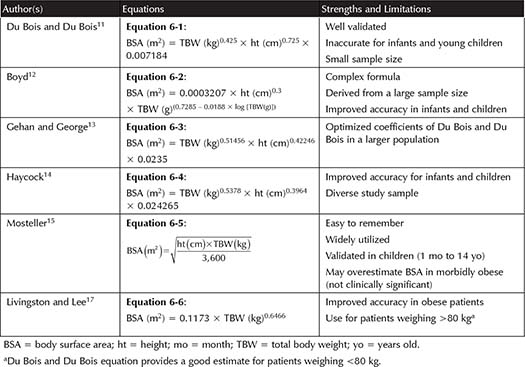
There were no modifications in the above equations until Mosteller devised an easy to remember modification of the Gehan and George equation (Equation 6-5, Table 6-1).15 Some say the Mosteller equation underestimates BSA in neonates and infants; however, other studies have validated the Mosteller equation in children between 1 month and 14 years of age.16 Mosteller is preferred because it is convenient to use and there were no clinically significant differences across participants.10
With all of the equations discussed so far, there has been great variability in results for obese patients. The Mosteller equation has been shown to slightly overestimate BSA, and the Du Bois and Du Bois equation has been shown to underestimate BSA as much as 20%, in obese patients.10,17 There does not appear to be a clinically significant difference, however, in the results achieved with the available equations.10
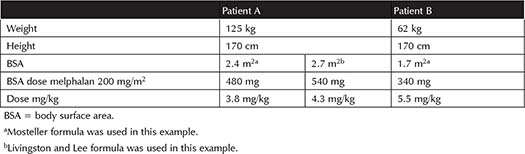
Livingston and Lee developed an equation to predict BSA in patients weighing >80 kg (Equation 6-6, Table 6-1).17 Forty-five patients weighing 51.3 kg to 248.6 kg, of which six were obese and 23 were morbidly obese (BMI range 18.3 to 91.3 m2), as well as the same sample used by Gehan and George were included in this study. Of note, height was eliminated because weight does not increase proportionately to height in obese patients. Table 6-2 shows the difference in the mg/kg dose when patients receive identical mg/m2 doses utilizing different BSA equations. This example demonstrates a 13% difference in BSA with the Mosteller equation compared to Livingston and Lee in a morbidly obese patient. It is important to note that as BSA increases, the mg/kg dose decreases.18 No literature supports adjusting the body weight based on ideal body weight (IBW) in the BSA equation or averaging two BSAs based on IBW and TBW.7 Other less validated strategies, such as BMI-adjusted BSA and computer modeling, are under investigation.19,20 See Table 6-1 for a list of all the equations discussed.
Table 6-2. Comparison of mg/kg Dose of Melphalan 200 mg/m2 in an Obese Patient and Normal-Weight Patient
Helpful Tips 
•Use TBW for BSA calculation, unless specified otherwise in the protocol.
•Mosteller is considered by many to be the gold standard and is widely utilized.
•As BSA increases, the mg/kg-dose decreases.
Summary 
•There are minimal, if any, clinically significant differences between available BSA formulas. As such, there is no single preferred formula.
•For patients weighing >80 kg, a case could be made to use the Livingston and Lee formula.
Alternatives to Body Surface Area Dosing
The goal of normalizing medication doses based on BSA or weight is to decrease interpatient variability. Normalizing has been shown, however, with select drugs such as busulfan and paclitaxel to not correlate well with studied pharmacokinetic parameters.21 Some have proposed an adaptive dosing strategy (prime dose, modified dose, and toxicity-adjusted dose) where the chemotherapy dose is adjusted based on pharmacokinetics and observed toxicity.22 For example, a busulfan test dose is used at some institutions to adjust the dose in the preparative regimen for stem cell transplantation (SCT).23 This allows for use of patient-specific pharmacokinetic parameters to better achieve an optimal AUC. In dose-adjusted EPOCH (etoposide, vincristine, and doxorubicin for 96 hours with bolus doses of cyclophosphamide and oral prednisone), the doses are increased or decreased by 20% each cycle depending on patients’ nadir platelets and absolute neutrophil count, allowing for greater dose individualization.24
Fixed-dose chemotherapy has been shown to be safer and more cost effective compared to BSA dosing.21 This method does not account for interindividual variability of drug exposure, leading to the practice of dose banding (where only several doses would be available for a given drug instead of dosing being further individualized to the patient). Dose banding has demonstrated greater precision in achieving a target AUC compared to flat-fixed dose.25
Lastly, capping doses has been implemented for certain medications like vincristine to prevent neurotoxicity.26 Some drugs, such as carfilzomib, remain in the central compartment and have a cap on BSA because the drug is not widely distributed and thus mimics the volume of distribution (Vd) of a normal-weight person.27 Obese patients with lung, colorectal, or hormone-refractory prostate cancer experienced less myelosuppression and were able to tolerate more cycles of chemotherapy, compared to nonobese patients, when their doses were capped at 2.2 m2.28 Some have argued, however, that capping may result in underdosing certain patients and suggest this practice be avoided, especially when the treatment intent is curative.
Specific Evidence by Cancer Type
Solid Tumors
Breast Cancer
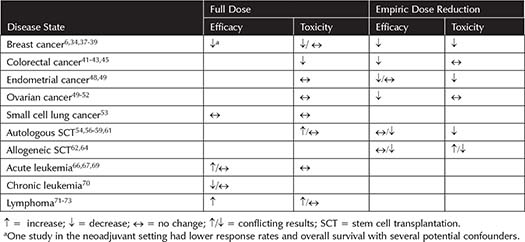
Obesity alone is an independent risk factor for breast cancer because of the persistent inflammatory cytokines and increased estrogen production by excess adipose tissue.29,30 Obese women with breast cancer have a 33% greater risk of death compared to nonobese breast cancer patients.31 It is well established that obese women are given empiric chemotherapy dose reductions by their oncologists.32,33 The data clearly support dosing on TBW compared to capping or dose reducing.6 See Table 6-3 for a complete summary of efficacy and safety outcomes.
In the neoadjuvant setting, overweight and obese patients receiving full dose chemotherapy have been shown to have worse pathologic complete response and overall survival (OS).34 Chemotherapy dose could contribute to the poorer outcomes, but there are other factors such as the difficulty in achieving negative margins and surgical complications in obese patients, which likely play a role. In the adjuvant setting, reducing doses has demonstrated worse outcomes for obese patients.6,35-37 Neutropenic fever episodes were found to decrease as BMI increases, as were treatment delays for myelosuppression.37,38 Less hematologic toxicity was found in patients with the highest BMI of patients receiving adjuvant CMF (cyclophosphamide, methotrexate, and fluorouracil).39 Other studies found no differences in toxicity.6 In the metastatic setting, decreased dose intensity decreased response rates in both obese and nonobese patients.40
Table 6-3. Efficacy and Toxicity Outcomes of Full-dose Versus Dose-reduced Chemotherapy
Colorectal Cancer
Obesity has not been associated with increased toxicity in the treatment of adjuvant colon cancer.41 Obese rectal cancer patients with stage II and III disease had a lower rate of leukopenia, neutropenia, and stomatitis compared to normal-weight individuals.42 Dose reductions in obese patients with metastatic colorectal cancer did not translate into reduced toxicity, and patients receiving full-dose chemotherapy did not experience more toxicity.43 In addition, obese patients with metastatic colorectal cancer receiving dose reductions had significantly worse progression-free survival (PFS).43 Obese colorectal cancer patients with liver metastases are at an increased risk for complications following surgery.44
Colorectal carcinogenesis has been linked to insulin resistance by activation of oncogenetic proteins, such as vascular endometrial growth factor, leading to potential resistance to certain targeted therapy such as bevacizumab and cetuximab.45 OS was statistically significantly improved in obese patients (n = 96) who received chemotherapy alone (versus chemotherapy plus targeted therapy) for advanced colorectal cancer.45 Obese patients appear to not respond as well to bevacizumab because those patients had a worse OS compared to normal-weight individuals.46 No significant differences were found with morbidly obese patients (BMI ≥35 kg/m2); however, the sample size was small (n= 82 patients).
Gynecological Cancer
Similar to breast cancer, obese patients are at an increased risk for endometrial cancer secondary to the estrogen created by adipose tissue. Morbidly obese patients with endometrial cancer were at an increased risk for death, compared to normal-weight patients.47 The impact of BMI was investigated for patients receiving cisplatin and doxorubicin for the treatment of endometrial cancer.48 In this study, 46% of drug doses were capped in 117 patients with a BSA >2 m2. No statistically significant associations with worse prognosis were found (HR 1.46, 95% CI 0.89 to 2.41); however, the study was not adequately powered to detect a difference. Morbidly obese patients had a shorter OS but no difference in PFS. As BMI increased hematologic toxicities decreased, suggesting dose capping may have contributed to the decline in OS.
One small study of obese patients with endometrial or ovarian cancer receiving paclitaxel and carboplatin showed no difference in toxicity between those who received full-dose chemotherapy versus capped dose.49 Dose reductions were more common in obese patients (n = 70) with advanced ovarian cancer in another study, leading to a worse PFS (11 versus 15 months, p = 0.04).50 A different study showed obese patients with ovarian cancer who received full doses of carboplatin and taxane chemotherapy had no worse PFS or OS.51 It is important to note that this study did not allow any estimates of creatinine clearance. In another study of patients with stage III or IV ovarian cancer who received lower dose intensity paclitaxel, disease-free survival and OS were significantly worse in obese patients.52
Limited data exist on the effects of obesity in patients with lung cancer. Survival and toxicity between obese and nonobese patients were compared in those with limited or extensive stage small cell lung cancer.53 Of the 262 patients, 27% were obese (females BMI ≥27.3 kg/m2 and males BMI ≥27.8 kg/m2), and 11% were severely obese (females BMI ≥32.3 kg/m2 and males BMI ≥31.1 kg/m2). There were no differences found in OS or toxicity when TBW was used for calculating chemotherapy doses. The study was not powered to detect differences in survival.
Hematologic Cancers
Stem Cell Transplantation
In the SCT setting, higher doses of chemotherapy are used. This may alter the pharmacokinetics, as some of the drugs utilized lose their linear dose response at higher doses.54 For example, protein binding can become saturated or altered at higher concentrations. Hematologic toxicities may not be as much of a concern in this population as other toxicities such as veno-occlusive disease or pulmonary and neurologic toxicity because patients receive stem cell support. Obesity in SCT recipients was associated with a significantly greater risk of acute graft-versus-host disease (GVHD) and infection, compared to patients with a normal BMI.55
Several studies have investigated the impact of obesity in patients undergoing autologous SCT. Obesity has been found to be a risk factor for worse outcomes.56 Greater relapses have been documented in obese patients with empiric dose adjustments made compared to TBW-based dosing, suggesting dose adjustments underdose patients.54,57 Tarella and colleagues found obese patients tolerated treatment better with trends of fewer transfusions and decreased duration of fever episodes, but statistically significantly worse event free survival (EFS) and OS. Dose reduction did occur in six out of the nine patients with BMI ≥32 kg/m2 in this study.56 In fact, obese patients (BMI >28 kg/m2) were at a 2.9-fold increased risk of death. Others have found similar results with greater treatment-related mortality in obese individuals, suggesting the need for utilization of an adjusted body weight (if TBW >15 kg above IBW, use 40% adjustment between TBW and IBW).58
Navarro and colleagues studied a large cohort of patients with Hodgkin or NHL (n = 4,681) and found no difference in OS between obese and nonobese participants.59 Patients in this study were dosed in a variety of ways (adjusted- or TBW-based dosing) depending on institutional standards. These OS results were confirmed in a similar cohort of lymphoma patients; however, obese patients had a lower 100-day mortality compared to those with a BMI ≤24.9 kg/m2 (p = 0.04).60 This study used an adjusted body weight (25% of the difference between IBW and TBW), suggesting no change in efficacy with reduced doses in obese patients. Bacterial infections, time to engraftment, and median hospital length of stay did not differ between the groups.
In another study, efficacy and toxicity outcomes were investigated for a large cohort of patients undergoing autologous SCT for multiple myeloma.61 Of the 1,087 patients, 198 were obese (BMI 30 to <35 kg/m2) and 125 were morbidly obese (≥35 kg/m2). No difference in PFS or OS in obese patients receiving single-agent melphalan was found compared to normal-weight individuals (p = 0.65). Obese patients receiving melphalan and total body irradiation (TBI) had an improved survival compared to normal-weight individuals (p = 0.005). Fortunately, TBI is no longer the mainstay of therapy as melphalan alone is preferred because of reduced toxicity. Dose reductions were statistically significantly greater in the obese group (p <0.0001); about 44% of obese and 22% of morbidly obese patients received at least 90% of the TBW-based dose. This reduction in dose did not significantly alter outcomes further confounding available data regarding the effect of obesity on drug dosing in the setting of autologous SCT.
In the allogeneic SCT setting, there are conflicting results as well. Patients weighing more than their IBW with chronic myeloid leukemia receiving cyclophosphamide and TBI- containing preparative regimen were found to be at a 60% increased risk of death.62 Another study in patients with acute or chronic leukemia confirmed these results that obese patients had worse relapse and nonrelapse survival.63 TBW was used to calculate chemotherapy doses in the former study, whereas IBW was used in the latter trial. A more recent study found a nonsignificant trend toward improvement in OS at 1 year in patients with a BMI >25 kg/m2 (31 months versus 39 months, p = 0.06).64 More deaths occurred earlier with greater hospital length of stay and more infections in the higher BMI group. Less acute and chronic GVHD occurred in the obese group but did not reach statistical significance. Heterogeneity between dosing strategies was noted, with doses being capped at 2.2 m2 regardless of actual BSA.
Overall, the data are contradictory leading to regimen and protocol-specific determinations in doses. The doses within protocols varied as well as therapeutic agents examined so it is difficult to draw sound conclusions. The more recent studies indicate no difference in efficacy and toxicity, possibly suggesting enhanced supportive care in obese patients over time (especially in the management of infectious complications).
Acute Leukemia
The leukemic cell microenvironment appears to be protected from vincristine, L-asparaginase, dexamethasone, daunorubicin, doxorubicin, and nilotinib by adipocytes.65 In a study of patients with acute myeloid leukemia (AML), there was no difference in OS when dosing chemotherapy on BSA using TBW (p = 0.829).66 No differences in pulmonary toxicities, cardiac toxicities, and infections were found between the obese and normal-weight groups. Another study found no difference in complete remission rates in AML patients, including 85 obese patients, treated with cytarabine and an anthracycline (p = 0.321).67 Doses in this study were calculated on TBW. Interestingly, when adjusted for confounding variables, OS was significantly shorter for normal-weight individuals compared to obese patients (p = 0.006).
There are limited data in obese patients with acute promyelocytic leukemia (APL). Higher BMI was associated with an increased risk for APL in patients with AML.68 In 343 obese preteenagers and adolescents with acute lymphocytic leukemia, greater relapses were found unrelated to dose (all based on TBW) and intervals between chemotherapy cycles.69
Chronic Leukemia
Limited data exist on tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia (CML) in obese patients. In those with a BMI >25 kg/m2 (max BMI 40 kg/m2), imatinib showed a longer time necessary to achieve complete cytogenetic response.70 Higher doses of imatinib, such as 600 mg daily, helped obese patients achieve more optimal responses. No differences were found in time to response with nilotinib, when comparing underweight/normal and overweight/obese patients, in a small cohort of 35 participants.70
Lymphoma
A study in NHL patients investigated the impact of obesity on disease outcomes, with the exception of vincristine.71 Vincristine is routinely capped at 2 mg to minimize neurotoxicity.26 Of the 712 patients, 23% were obese (BMI ≥30 kg/m2). Overweight and obese patients trended toward increased survival compared to nonobese participants. The number of documented infections was significantly higher in the obese group. Similar survival results were found in obese patients with Hodgkin lymphoma, with no difference in relative treatment intensity.72 More recently, a large study of 1,241 patients (326 obese patients) with diffuse large B-cell lymphoma found that obese patients experienced less febrile neutropenia and treatment-related mortality after receiving R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).73 There were no significant increases in the number of dose reductions with obese patients in this study.
Helpful Tips 
•Most of the studies examining the impact of obesity on cancer and/or supportive care outcomes are retrospective, with limited power to detect significant differences between groups.
•First-cycle dose intensity was used as an endpoint for many included studies, but not for the entire course of treatment because dose reductions could occur for a variety of reasons.
Summary 
•In breast cancer, abundant data support worse outcomes with dose reductions for obese patients, especially in the adjuvant setting. As such, obese patients with breast cancer should be dosed on TBW.
•No increase in toxicities has been reported in obese patients with AML or colorectal, endometrial, ovarian, or lung cancer. Studies in obese patients with lymphoma, however, have shown varying results.
•A variety of dose adjustments in chemotherapy administered prior to SCT have been studied for obese patients. Overall, results tended to not show more toxicity with TBW-based dosing, but it is important to follow specific protocol dosing recommendations.
Antineoplastic Medications
Alkylating Agents
Busulfan
Busulfan is commonly used as part of the preparative regimen for SCT. In the SCT setting, clearance was significantly elevated by 17% in obese patients (BMI 27 to 35 kg/m2) and by 32% in those morbidly obese (BMI >35 kg/m2).74 When clearance was normalized to an adjusted body weight [(25% adjusted body weight = IBW + 0.25 (TBW – IBW)] or BSA, there was no difference in drug clearance. Busulfan dosing on IBW for obese and overweight individuals demonstrated a lower AUC as compared to normal-weight patients.75 Despite this, EFS and OS did not appear to differ (p = 0.37 and p = 0.26, respectively). Two studies, one with 26 obese patients undergoing autologous SCT and another with 39 obese patients (BMI 27 to 35 kg/m2) and 11 morbidly obese (BMI >35 kg/m2) undergoing autologous or allogeneic SCT found that use of a 25% adjusted body weight minimized differences in clearance between obese and nonobese participants.76,77
Significant interpatient and disease variability has been noted, especially for patients with NHL where clearance decreased by 32%, in a study utilizing oral busulfan.74 Intravenous (IV) busulfan use has been shown to minimize interpatient variability.78 Pharmacokinetically-adjusted busulfan has resulted in similar rates of toxicity and OS, regardless of the route of administration (IV versus oral).79 In one study of pediatric SCT patients, busulfan test doses were administered and obese participants had the lowest clearance (thus requiring lower busulfan doses).80 This emphasizes the need to closely monitor busulfan levels in patients, particularly those receiving busulfan doses >12 mg/kg. At this time, once-daily busulfan dosing strategies incorporating pharmacokinetic monitoring are being used.81 Patients weighing >120% of their IBW are dosed based on a 50% adjusted body weight using this dosing methodology.
Carmustine
Lymphoma patients have been given carmustine as part of a preparative regimen for autologous SCT.82 In this study, a 25% adjusted body weight was used to calculate BSA for obese patients. See the melphalan section for outcomes specific to carmustine because some were not reported. In another study, patients with a history of breast cancer were given a carmustine preparative regimen.83 Patients who were >20% of their IBW were dosed using an average of their IBW and TBW to calculate BSA. The median AUC was not significantly different in obese compared to nonobese patients. However, there was a greater than 10-fold interpatient variability (ranges were not reported for the obese group).
Cyclophosphamide
In a small sample of patients with breast cancer, the half-life of cyclophosphamide was increased and clearance was lower, with no change in the Vd, for patients with increased body weight.84 High-dose cyclophosphamide has been linked to heart failure so it is important to calculate the correct dose to avoid excessive toxicities.85 One case report by de Jonge and colleagues of an obese patient who underwent autologous SCT had a 9% increase in exposure of cyclophosphamide (used in the conditioning regimen).86 However, the AUC of the active metabolite 4-hydroxycyclophosphamide was increased by 94% secondary to increased hepatic enzyme activity in obese patients. The authors suggested using a 40% adjusted body weight in obese patients. Another study by Petros and colleagues used an adjusted dose for those >20% of their IBW (n= 25) by averaging patients’ BSA calculated using IBW and TBW.83,86 The former study by de Jonge found that systemic exposure was decreased and suspected under treatment. It is important to note that the 4-hydroxycyclophosphamide active metabolite was not measured in this study. Two additional studies used a 25% adjusted body weight for patients who were >20% of their IBW.76,87 One of these studies, with patients weighing up to 132 kg, found patients with a BMI >25 kg/m2 had more mucositis.87 This was attributed to the longer half-life of cyclophosphamide in obese patients.
Ifosfamide
In a small study of obese patients with non-small-cell lung cancer receiving ifosfamide, patients were found to have no difference in the clearance of ifosfamide but an increased Vd, leading to a prolonged half-life.88 This increase in Vd suggests that ifosfamide is able to distribute into adipose tissue and that TBW may be the best weight descriptor to achieve adequate drug concentrations. No study has evaluated optimal ifosfamide dosing for obese patients. It is important to clinically evaluate the patient’s muscle mass as compared to adipose tissue, because ifosfamide appears to distribute well into adipose tissue. The extended half-life seen in obesity can increase the amount of toxicities. Adjustments have not been studied but may be reasonable for those patients for whom there is concern for greater toxicity.
Mechlorethamine
Mechlorethamine enters the cell rapidly by an active chlorine transporter.89 It is rapidly cleared from the systemic circulation so pharmacokinetic parameters have not been characterized. The product labeling recommends using an IBW (“dry weight,” mg/kg).90 The most common use of this agent, however, is in the MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) regimen utilizing BSA-based dosing. Therefore, obese dosing recommendations cannot be made. It is important to consider the impact of edema and ascites when assessing a patient as well, as that could alter mechlorethamine exposure.91
Melphalan
Patients with relapsed diffuse large B-cell lymphoma were given melphalan as part of a preparative regimen containing etoposide and cytarabine prior to autologous SCT.82 A 25% adjusted body weight was used to calculate BSA for patients who were greater than their IBW. Patients who received >3.6 mg/kg of melphalan based on TBW had more mucositis and a longer hospital stay (p = 0.001 and p = 0.04, respectively). There was no correlation between dose:weight ratio and relapse or survival outcomes. Patients with a higher BSA had less mucositis and shorter hospital length of stay. In another study, patients weighing up to 135 kg were dosed on BSA using TBW (max BSA = 2.7 kg/m2).18 Obese patients had less mucositis. This was confirmed in another study where patients weighing >60 kg received melphalan based on a 40% adjusted body weight (maximum weight = 160 kg; maximum BMI = 55 kg/m2).92 As BSA increased, mg/kg-dose decreased as did the rate of mucositis. This would suggest that TBW should be used to dose melphalan.
Procarbazine
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


