regulation of plasma electrolyte concentrations and fluid balance,
Of these, a basic knowledge of the mechanisms of electrolyte and fluid handling by the kidney is essential for understanding the uses and unwanted effects of diuretics.
The kidney and maintenance of salt and water balance
Each day the renal glomeruli of a healthy adult filter about 180 L of fluid (about 20% of the plasma that enters the glomerular capillaries), together with its content of ions such as Na+, K+ and Cl–. Since the urine output is only 1–2 L per day, it is clear that most of the filtered fluid is absorbed back from the tubule into the blood. Different regions of the tubule and collecting duct vary in their capacity to reabsorb water and solutes (Figs 14.1 and 14.2).
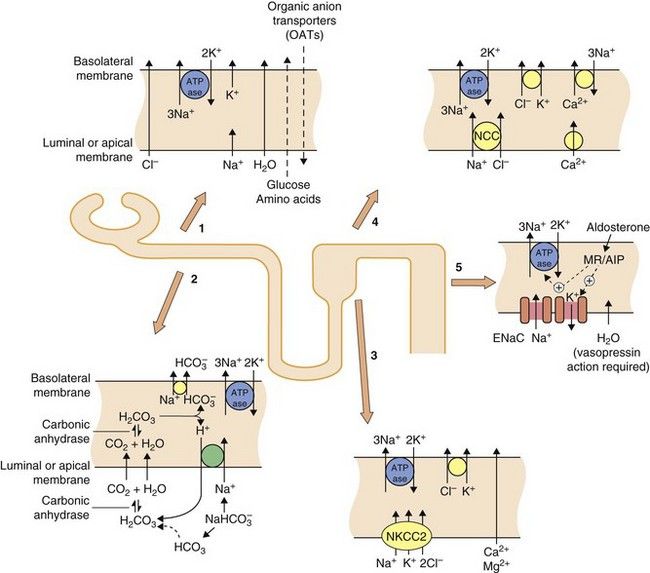
Fig. 14.1 Transport mechanisms for solutes in the kidney.
In all segments of the renal tubule there is active transport of Na+ out of and K+ into the cell across the basolateral membrane using Na+/K+-ATPase proton pumps. This sets up electrochemical gradients for the transport of other ions. In the proximal tubule (sites 1 and 2), considerable amounts of Na+, glucose and amino acids are taken up from the lumen, along with water which crosses via aquaporin channels. The principal function of the organic anion transporters (OATs) (site 1), including OAT1 and OAT3 (Ch. 2) is the elimination of metabolites of ingested xenobiotics. Transport by OATs also enables diuretic drugs such as furosemide and bendroflumethiazide to gain access to their sites of action on apical membranes in the tubule. Hydrogen ions are excreted in exchange for Na+ uptake and this, in part, depends upon the activity of carbonic anhydrase (site 2). In the ascending limb of the loop of Henle (site 3), the luminal membrane has a Na+/K+/2Cl− co-transporter (NKCC2) but is impermeable to water. In the proximal part of the distal tubule (cortical diluting segment; site 4) Na+ and Cl− ions are reabsorbed by the Na+/Cl− co-transporter (NCC), but water is not reabsorbed. Ca2+ also exchanges with three Na+ at the basolateral border at this site. In the distal part of the distal tubule and collecting duct (site 5) Na+ is reabsorbed from the lumen via an epithelial Na+ channel (ENaC), in exchange for loss of K+ into the lumen. The expression and activity of ENaC and the basolateral Na+/K+-ATPase pump are regulated by aldosterone acting via mineralocorticoid receptors (MRs) and aldosterone-induced proteins (AIPs). Water is reabsorbed in the collecting duct under the influence of antidiuretic hormone (ADH, vasopressin) acting through vasopressin receptors in the basolateral membrane.
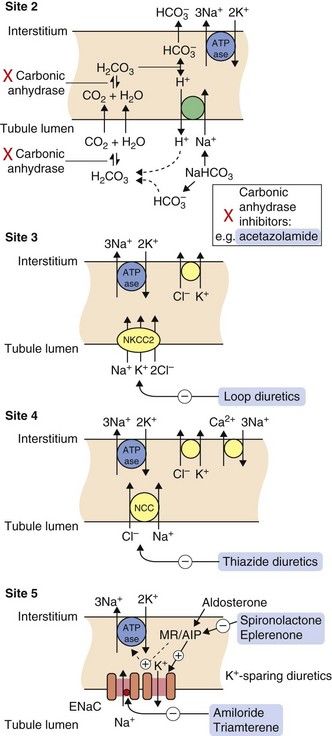
Fig. 14.2 Sites of action of diuretics.
For location of these sites in the tubule, see Fig. 14.1. Osmotic diuretics increase osmotic pressure through the tubule, reducing electrolyte reabsorption across the luminal membrane. Other drugs gain access to their sites of action after secretion into the tubule by the organic anion transporters (OATs) in the proximal tubule. Acetazolamide inhibits carbonic anhydrase (site 2) and is a weak self-limiting diuretic, now largely used for other conditions such as glaucoma. Loop diuretics such as furosemide block the luminal Na+/K+/2Cl− co-transporter (NKCC2) and inhibit up to 25% of filtered Na+ reabsorption (site 3). The thiazide diuretics inhibit the luminal Na+/Cl− co-transporter (NCC) (site 4) and can reduce reabsorption of 5–8% of filtered Na+. The potassium-sparing diuretics spironolactone and eplerenone compete with aldosterone for the mineralocorticoid receptor (MR), blocking the induction by aldosterone of the expression and activity of the epithelial Na+ channel (ENaC), the basolateral Na+/K+-ATPase pump and other aldosterone-induced proteins (AIP) (site 5). Amiloride and triamterene act directly on ENaC to block Na+ reabsorption. Potassium-sparing diuretics inhibit the reuptake of less than 3% of filtered Na+.
The proximal convoluted tubule
In the proximal convoluted tubule, about 65–70% of the filtered Na+ is reabsorbed together with an equivalent (isosmotic) amount of water. Therefore, on leaving the proximal tubule, the tubular fluid still has the same osmolarity as plasma. Reabsorption of ions from the proximal tubule into the renal tubular cells is passive (Fig. 14.1, site 1). The activity of the Na+/K+-ATPase pump on the basolateral surface of the tubular cell (transporting three Na+ out of the tubular cell in exchange for two K+) helps to establish the electrochemical gradient for passive Na+ reabsorption from the tubular lumen into the tubule cell. Water reabsorption by the proximal renal tubule is driven by the osmotic gradient across the tubular cells, which is created by the active transport of Na+ out of the cell across the basolateral membrane. Water reabsorption occurs via transmembrane aquaporin (AQP) channels. The extent of the proximal tubular reabsorption of Na+ and water is determined by two regulatory mechanisms: glomerulotubular feedback (enhanced tubular Na+ reabsorption when the glomerular filtration rate rises), and various neural and hormonal influences such as the sympathetic nervous system, angiotensin II, endothelin, dopamine and parathyroid hormone.
The proximal tubule has transporters for the secretion of organic anions into the tubular lumen (Fig. 14.1, site 1; see also Ch. 2), and the reabsorption of water-soluble essential nutrients, such as glucose and amino acids, from the lumen. The organic anion transporters (OATs) are important for the transport of many drugs and their metabolites from the blood into the tubule (e.g. see acetazolamide and loop diuretics below). The inward Na+ gradient provides the drive for several carriers, such as that for glucose. Bicarbonate is also reabsorbed from the proximal tubule by a mechanism dependent on the enzymatic activity of carbonic anhydrase (Fig. 14.1, site 2).
The loop of Henle
The descending limb of the loop of Henle is permeable to water, due to the presence of aquaporin channels, but not to Na+. Water passes from the tubule into the interstitium of the renal medulla, where the fluid is hypertonic as a result of ion transport in the thick ascending limb of the loop of Henle (see below). Therefore, tubular fluid reaching the ascending limb of the loop of Henle is hypertonic.
The thick ascending limb of the loop of Henle is impermeable to water but has an active Na+/K+/2Cl– co-transporter complex (NKCC2) in the luminal (apical) membrane (Fig. 14.1, site 3). Na+ is actively transported from the tubular cells to the interstitium by the Na+/K+-ATPase pump in the basolateral membrane. This creates a low intracellular Na+ concentration in the tubular cells and generates the Na+ ion gradient that drives the luminal NKCC2 co-transporter. The ascending limb of the loop of Henle can reabsorb up to 25% of the Na+ filtered at the glomerulus. K+ that is carried from the tubule into the cells of the loop by the NKCC2 co-transporter is recycled back into the tubular lumen, which ensures that there is always enough tubular K+ to continue to favour Na+ reabsorption. K+ recycling creates a lumen-positive transepithelial voltage gradient, which drives a paracellular ionic current that is responsible for half the total Na+ reabsorbed by this region of the kidney, along with Ca2+ and Mg2+.
The reabsorption of Na+, but not water, by the thick ascending limb of the loop of Henle establishes the hypertonicity of the medullary interstitium (the corticomedullary concentration gradient). This interstitial hypertonicity is responsible for an osmotic gradient across the collecting ducts, which permits the formation of hypertonic urine (see below). There are various hormonal regulators of Na+ reabsorption in the ascending limb of the loop of Henle, including calcitonin, parathyroid hormone and prostaglandin E2.
The proximal (cortical) diluting segment of the distal convoluted tubule
The filtrate leaving the loop of Henle is hypotonic and passes to the proximal part of the distal convoluted tubule (also known as the cortical diluting segment of the distal tubule). This part of the renal tubule is impermeable to water but has a luminal Na+/Cl– co-transporter (NCC) (Fig. 14.1, site 4). The driving force for this thiazide-sensitive co-transporter is again generated by the Na+/K+-ATPase pump in the basolateral membrane. About 5–8% of the filtered Na+ load can be reabsorbed at this site. The rich blood supply to this region allows rapid diffusion of the reabsorbed ions into the plasma and prevents the interstitium from becoming hypertonic. Reabsorption of Ca2+ is also regulated at this site, under the influence of parathyroid hormone and calcitriol (Ch. 42). The rate of Ca2+ transport is inversely related to that of Na+ transport; this is because Na+ inside the tubular cell either inhibits luminal voltage-gated Ca2+ channels or reduces the activity of the basolateral Na+/Ca2+ exchanger.
In the cortical diluting segment of the distal tubule the increased luminal concentration of Na+ or Cl– initiates two responses that limit Na+ loss. The first is tubuloglomerular feedback, a mechanism (possibly mediated by adenosine) that constricts the afferent glomerular arteriole to that nephron. The second is secretion of renin, which, through activation of the renin–angiotensin system, eventually enhances the release of aldosterone from the adrenal cortex and increases Na+ reabsorption at the distal part of the distal convoluted tubule (see Chs 6 and 44, and below).
The distal part of the distal convoluted tubule and the collecting duct
The tubular fluid that has become yet more hypotonic in the cortical diluting segment of the distal tubule is delivered to the distal part of the distal tubule and then to the collecting duct. There are two main cell types in this region, the principal cells and the intercalated cells.
In the principal cell, Na+ is reabsorbed through a highly specific amiloride-sensitive epithelial Na+ channel, known as ENaC, and this is accompanied by obligatory K+ loss into the urine (Fig. 14.1, site 5). Aldosterone acts at this site at cytosolic mineralocorticoid receptors (MRs), inducing transcription of genes encoding components of ENaC and the basolateral Na+/K+-ATPase pump. Other aldosterone-induced proteins (AIPs) include serum- and glucocorticoid-regulated kinases (SGK) and channel-inducing factor, which further increase the activity of ENaC and the Na+/K+-ATPase. Together, these changes increase the reabsorption of Na+ from the tubule and the concomitant loss of K+ into the lumen.
Less important hormonal regulators of Na+ reabsorption in the distal tubule and the collecting duct include calcitonin and bradykinin. The natriuretic peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), also decreases Na+ reabsorption by receptor-mediated phosphorylation of ENaC. Overall, only about 3–5% of filtered Na+ is reabsorbed at the distal part of the distal tubule. However, the distal renal tubule is the primary site in the kidney responsible for maintenance of K+ homeostasis. Relatively small changes in extracellular K+ concentration can affect cardiac muscle, skeletal muscle and brain function, so the extracellular K+ concentration is closely regulated.
The principal cell is also the site of action of antidiuretic hormone (ADH, vasopressin; Ch. 43). This hormone is secreted by the posterior pituitary gland and binds to receptors in the basolateral membrane, where it increases the permeability of the cell to water by upregulating aquaporin (AQP2) channels. In the presence of ADH, water reabsorption into the hypertonic medullary interstitium is increased, which concentrates the urine as it passes through the collecting duct.
Intercalated cells are the second important cell type in the distal part of the distal tubule and the collecting duct (not illustrated in Figs 14.1 and 14.2). Two subtypes of intercalated cells express H+-ATPases that help to regulate acid–base balance by secreting or reabsorbing H+ and HCO3−.
Diuretic drugs
Mechanism of action
Acetazolamide interferes with the small proportion of Na+ that is actively reabsorbed in the proximal tubule in exchange for H+ (Fig. 14.2, site 2). This process is dependent on carbonic anhydrase, which is inhibited by acetazolamide. Acetazolamide increases HCO3−, Na+ and K+ secretion, causing alkaline urine. H+ retention produces mild acidosis in the blood, but the fall in plasma HCO3− concentration stimulates carbonic anhydrase activity, which rapidly leads to tolerance to the diuretic action of acetazolamide. In consequence, acetazolamide does not have a clinically useful diuretic action. Its clinical use is restricted to treatment of altitude sickness (Ch. 13) and glaucoma (Ch. 50).
Pharmacokinetics
Acetazolamide is secreted into the proximal renal tubule by organic acid transporters (OATs) and works at the luminal surface of the proximal tubule. It is eliminated unchanged in the urine.
Osmotic diuretics
Mechanism of action
Mannitol is filtered at the glomerulus but not reabsorbed from the renal tubule. It exerts osmotic activity within the proximal renal tubule and particularly in the descending limb of the loop of Henle, and limits passive tubular reabsorption of water. Water loss produced by mannitol is accompanied by a variable natriuresis (up to 25% of filtered Na+). Unlike other diuretics, the osmotic action of mannitol produces an initial expansion of plasma and extracellular fluid volume, which limits its clinical uses.
Mannitol does not readily cross the blood–brain barrier. It is used to treat some forms of acute brain injury, when the main mechanism of action may be through haemodilution and reduced blood viscosity which may limit ischaemic damage, rather than a dehydrating action on cerebral tissues.
Pharmacokinetics
Mannitol is given by intravenous infusion and is excreted unchanged at the glomerulus. It has a half-life of 2 h, which is substantially increased in renal impairment.
Loop diuretics
Mechanism of action and effects
Loop diuretics, such as furosemide, must be secreted into the proximal kidney tubule by the tubular organic anion transporters to access their site of action. The extent of the natriuresis and diuresis is dependent on the rate of delivery of the drug to the renal tubule via this secretory mechanism. Once these transporters have been saturated, increasing the dose of the diuretic will not enhance its effect. Loop diuretics bind to the Na+/K+/2Cl− co-transporter complex (NKCC2) at the luminal border of the thick ascending limb of the loop of Henle, and inhibit Cl− reabsorption. This diminishes the electrochemical gradient across the cell and reduces Na+ reabsorption from the tubular fluid (Fig. 14.2, site 3). Loop diuretics therefore reduce the ability of the kidney to generate the medullary ionic concentration gradient and impairs generation of concentrated urine in the collecting duct. Loop diuretics also inhibit tubuloglomerular feedback and the afferent artery vasoconstriction in response to the increased tubular concentrations of Na+ and Cl−. They are powerful, ‘high-ceiling’ diuretics which can inhibit reabsorption of up to 25% of the Na+ that appears in the glomerular filtrate.
The dose–response curves of loop diuretics are steep, but the doses required to achieve maximal inhibition of Na+ reabsorption show wide inter-individual variation. Because they have a short duration of action, there is partial compensation for the natriuresis by subsequent rebound Na+ uptake from the tubular fluid after their action has finished. Loop diuretics remain effective even in advanced renal failure, but larger doses are necessary to deliver an effective concentration of drug to the remaining renal tubules as the reduced tubular secretion results in greater metabolism of the drug in the liver.
When injected intravenously, furosemide releases vasodilator prostaglandins, such as prostacyclin, into the circulation and produces a short-lived venodilation. Pooling of blood in these capacitance vessels reduces central blood volume, which can be useful in the treatment of acute left ventricular failure (Ch. 7). Loop diuretics also produce arterial vasodilation (see thiazide diuretics), but because of their short duration of action they are not widely used to treat hypertension, except in renal failure when their diuretic action can be useful.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree










