Distal Pancreatectomy with Splenic Preservation
Adam S. Brinkman
Sharon M. Weber
DEFINITION
Distal pancreatectomy with splenic preservation (DPSP) is the complete removal of the distal pancreas (lateral to the superior mesenteric vein/portal vein [SMV/PV] confluence) while preserving the spleen via meticulous dissection and preservation of the splenic artery (SA) and splenic vein (SV), or with sacrifice of these vessels (Warshaw technique) with preserved blood flow to the spleen through the short gastric vessels.
Indications for DPSP include benign tumors for which local excision is inadequate (neuroendocrine tumors), pancreatic cysts, chronic pancreatitis limited to the distal pancreas, and selected cases of distal pancreatic trauma. DPSP for distal pancreatic malignancies (adenocarcinoma) is controversial as body and tail lesions may have lymphatic drainage to the splenic hilum.
The majority of cases performed in the United States are accomplished either open or laparoscopically, whereas fewer are performed using robotic assistance or with a hand port. Variation in surgical technique with respect to preservation of the splenic vessels versus ligation of the vessels exists.
DIFFERENTIAL DIAGNOSIS
Premalignant or malignant lesions a DPSP may be considered:
Solid pancreatic tumors
Functional and nonfunctional neuroendocrine (NE) tumors
Cystic pancreatic tumors
Intraductal papillary mucinous neoplasms (IPMNs)
Mucinous cystadenomas (MCN)
Solid pseudopapillary tumors
Benign conditions
Congenital cysts
Serous cystadenomas (SCA)
Chronic pancreatitis pancreatic pseudocysts can also be theoretically resected with a DPSP
The location of the lesion and the anatomic relationships of the splenic vasculature are the major factors that should be evaluated before considering a DPSP as the surgical procedure of choice.
PATIENT HISTORY AND PHYSICAL FINDINGS
Patients being considered for DPSP should be evaluated with a thorough history and physical examination including a detailed past medical history, past surgical history (including previous foregut and pancreatic surgery), as well as personal and family history of pancreatic disease or malignancy.
Particular attention should be paid to symptoms of pancreatic insufficiency, nutritional status, and ability to tolerate a pneumoperitoneum of 15 mmHg if a laparoscopic approach is chosen.
Severely malnourished patients (more than 10% to 15% of ideal body weight [IBW] loss in the preceding 2 months) or serum albumin less than 3 g/dL should be considered for supplemental nutrition; enteral nutrition via Dobhoff tube or total parenteral nutrition through indwelling central venous catheter.
IMAGING AND OTHER DIAGNOSTIC STUDIES
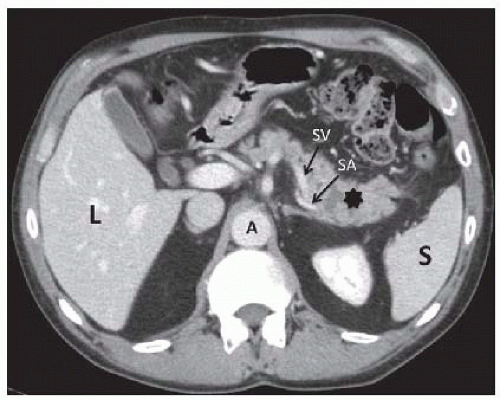
Pancreatic protocol helical computed tomography (CT) with oral and dual phase intravenous contrast is the preferred imaging modality for pancreatic diseases. The thin cuts through the pancreas allows for ideal delineation of vasculature, size of the lesion, involvement of surrounding structures, and evidence of local disease progression (FIG 1). Careful attention should be paid to the arterial and venous phases as well as the arterial blood flow to the spleen, as this can determine feasibility of splenic preservation.
The relationship of the mass, cyst, or stricture should be noted and its anatomic relationship to the SV, inferior mesenteric vein (IMV), and SA should be noted in anticipation of potential intraoperative pitfalls.
In addition to CT, consideration can also be given to endoscopic ultrasound (EUS) to better evaluate the pancreatic lesion and its relationship to the surrounding structures. In addition, sampling of the mass with fine needle aspiration for diagnostic purposes or aspiration of a cyst fluid for evaluation of carcinoembryonic antigen (CEA), amylase concentration, and mucin analysis may assist with diagnosis and operative planning. The presence of local disease discovered on CT imaging may be further investigated with EUS and suspicious masses (often lymph nodes) can be sampled.
Additional pathology can also be identified, including isolated gastric varices indicative of SV thrombosis and sinistral portal hypertension.
Additional diagnostic testing that may be considered prior to a DPSP includes magnetic resonance imaging (MRI) or magnetic resonance cholangiopancreatography (MRCP), which may better define the pancreatic ductal anatomy.
Consideration can also be extended to endoscopic retrograde cholangiopancreatography (ERCP), the gold standard to define pancreatic ductal anatomy, which also offers an opportunity for evaluation of communication with the main pancreatic duct for cystic lesions, which is diagnostic for IPMN.
Additional imaging including a chest radiograph or chest CT scan may be obtained for complete staging and preoperative evaluation in patients with suspected malignancy.
SURGICAL MANAGEMENT
Preoperative Planning
Prior to proceeding to the operating room (OR), all patients should be evaluated with a full history and physical examination with cardiovascular and pulmonary testing as medically needed. Patients scheduled to undergo a laparoscopic distal pancreatectomy should be made aware of the potential need to convert to an open approach during the operation.
Consideration should also be given to vaccinating against encapsulated bacteria including Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae.
These vaccines should be given 1 to 2 weeks prior to operative intervention in the event splenic preservation is not possible.
Should a splenectomy be required in a nonvaccinated patient then vaccines should be administered within 2 weeks following surgical intervention to mitigate postsplenectomy sepsis.
Patients should also obtain baseline laboratory function including type and screen, serum electrolytes, hemoglobin and hematocrit as well as pregnancy testing in reproductive-aged females.
Preoperative antibiotics and deep venous thrombosis (DVT) prophylaxis should be ordered prior to patient arrival to the OR. Arterial lines and central venous catheters are placed selectively for high-risk patients.
Positioning
Patients should be positioned supine on the OR table with arms extended wide with easy access to the OR table should a Bookwalter retractor need to be placed. Preoperative DVT prophylaxis with heparin should be administered subcutaneously prior to the induction of general anesthesia, bilateral lower extremity sequential compression devices should be placed, and a single dose of a second-generation cephalosporin should be administered within 60 minutes of skin incision.
After the patient is intubated, a Foley catheter should be placed under sterile technique and a nasogastric tube should be placed for gastric decompression.
The abdomen is shaved, prepped, and draped, with the xiphoid process, umbilicus, and right and left anterior superior iliac spines exposed.
An adequate prep will allow for easy incision planning should there be a need to convert to the open technique.
Some surgeons prefer a semilateral positioning in a relaxed right lateral decubitus position to assist with visualization of the left upper quadrant, whereas others prefer a split leg (Nissen) table to allow the surgeon to stand between the patient’s legs.
Standard laparoscopic instruments (including 0- and 30-degree laparoscopes), ultrasonic dissector or bipolar electrocautery, laparoscopic staplers (vascular and bowel staple loads), and clip appliers should be available.
TECHNIQUES
LAPAROSCOPIC APPROACH
Port Placement
Access to the abdominal cavity can be accomplished with either a supraumbilical Veress needle or Hassan trocar depending on surgeon preference and a pneumoperitoneum is established at 15 mmHg.
Three to four additional ports are placed under direct visualization, usually with one in the upper midline or just right of midline, depending on the patient’s anatomy. Two additional ports (a 5 mm and a 10 mm to accommodate the laparoscopic stapler) can be placed in the left middle or lower quadrant (FIG 2).
Once all ports are placed, a thorough diagnostic laparoscopy is performed to rule out metastatic disease. If abnormal lesions are identified, they should be biopsied and sent for frozen sectioning. Adhesions are freed to allow full visualization of the left upper quadrant.
Opening the Lesser Sac
The lesser sac is then opened along the greater curvature of the stomach inferior to the gastroepiploic vessels through the gastrocolic ligament. This allows visualization of the posterior stomach and the anterior surface of the pancreas (FIG 3).
The short gastric vessels along the superior aspect of the fundus should be preserved, as they may be required for splenic preservation if the SA and SV are divided (Warshaw technique).
If splenectomy is planned, all short gastric vessels will need to be transected.
Identification of the Pancreatic Lesion
Every effort should be made to identify or palpate the pancreatic lesion and its relationship to the pancreatic neck. Lesions amenable to a DPSP are those to the left of the SMV/PV confluence (FIG 4). If the lesion cannot be identified then employment of the laparoscopic ultrasound or EUS can be performed to assist with localization.
Dissection of the Pancreas
Dissection along the inferior border of the pancreas begins above the ligament of Treitz and should be extended toward the splenic hilum.
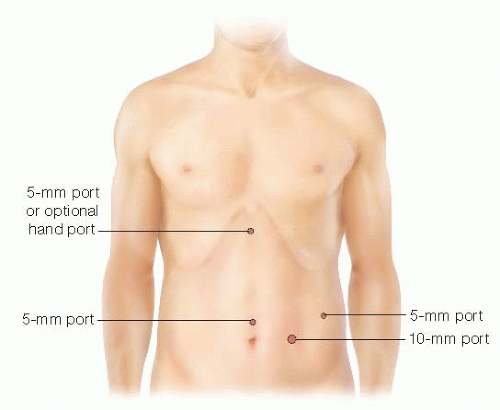
FIG 2 • Port placement for a laparoscopic DPSP includes three 5-mm ports, one 10-mm port, and an occasional hand port placed in the upper midline to assist with specimen retraction and dissection.
Careful attention is paid to opening just the thin peritoneum inferior to the pancreas and to elevate the pancreas from the retroperitoneal tissue by assuring the plane of dissection stays within the fibroareolar plane. If this is done correctly, the SV will usually be elevated with the pancreas and will result in mobilization of the pancreas anteriorly (FIG 5).
Careful attention is required to avoid lacerating or transecting the posterior SV.
The IMV should be identified and ligated if it enters the SV to the left of the mass or preserved if it enters the SV to the right of the planned pancreatic transection plane. Neoplastic involvement of the IMV requires ligation and resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree