The hypothalamus
The hypothalamus is derived from forebrain tissue on either side of the third ventricle and links the nervous system to the endocrine sytems through the pituitary gland. The hypothalamus is a complex region in the brain that co-ordinates many behavioural and circadian rhythms in addition to enforcing homeostatic mechanisms, on specific target glands. The hypothalamus must respond to many different external and internal signals by releasing a variety of factors that act on the pituitary. In order to accomplish such a complex process the hypothalamus is connected with many parts of the CNS (e.g. stress results in release of corticotrophin-releasing hormone (CRH)).
The hypothalamus secretes a number of hormones and other chemical agents that pass down the hypothalamo-hypophyseal portal blood vessels to the pituitary where they regulate the release of anterior pituitary hormones. The hormones produced by the target glands controlled by the anterior pituitary may exert negative feedback effects on the secretion of the corresponding hypothalamic or pituitary hormone. For example, plasma [free cortisol] primarily influences the output of hypothalamic CRH while plasma [free thyroxine (FT4)] inhibits the release of TSH from the pituitary. Table 7.1 list the various hypothalamic and pituitary hormones together with the factors that regulate their release. Concentrations of hypothalamic hormones in peripheral blood for the most part do not reflect hypothalamic acyivity and are not considered to be of clinical relevance.
Figure 7.1 Factors which regulate the release of anterior pituitary hormones.
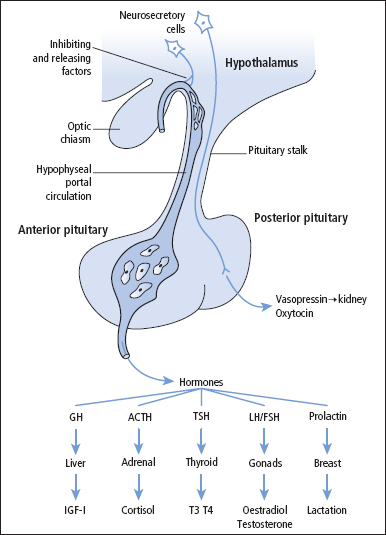
Table 7.1 Hypothalamic and anterior pituitary hormones and factors
| Hypothalamic-stimulating hormone | Anterior pituitary hormone(s) released | Feedback control hormone or compound |
| CRH | ACTH, β-lipotrophin (LPH) | Cortisol |
| GHRH, GnRH | GH | Somatostatin |
| FSH | Gonadal steroids and inhibin | |
| GnRH | LH | Gonadal steroids |
| No stimulating factor identified | Prolactin | Dopamine |
| Thyrotrophin-releasing hormone (TRH) | TSH (and prolactin) | FT4, FT3 |
Anterior pituitary hormones
The pituitary gland comprises the embryologically and functionally distinct anterior pituitary (adenohypophysis) and posterior pituitary (neurohy pophysis). The anterior pituitary hormones and their corresponding regulatory hormones/factors are described in detail below.
Corticotrophin-releasing hormone and adrenocorticotrophic hormone
Hypothalamic CRH is composed of 41 amino acids, and is the main stimulatory factor involved in the control of the pituitary–adrenal axis. Its release is subject to negative feedback control by [free cortisol] released from the adrenal cortex (Figure 9.2).
ACTH is a monomeric polypeptide hormone of 39 amino acids secreted by cells known as corticotrophs. The biological activity of ACTH is contained in the 24 amino acids at the N-terminal end. The major activity of ACTH is the stimulation of adrenal steroid synthesis, especially that of the glucocorticoid, cortisol, but it also plays a permissive role in the synthesis of aldosterone. ACTH also stimulates melanocytes to produce melanin. This is the cause of the increased pigmentation seen in patients with ACTH-driven causes of Cushing’s syndrome, in adrenocortical hypofunction and in Nelson’s syndrome, a rare disorder arising from an ACTH-secreting pituitary macroadenoma after a therapeutic bilateral adrenalectomy. ACTH shows a marked diurnal variation, with the highest concentrations being found at approximately 8 am and the lowest at midnight. Marked increases in ACTH also occur with stress. ACTH is unstable in blood or plasma unless frozen. Blood samples should be collected in EDTA on ice and the plasma separated within 30 min and then frozen immediately.
ACTH and β-lipotrophin (β-LPH) exist next to each other at the C-terminal end of a much larger precursor molecule, pro-opiomelanocortin. In ectopic ACTH production, it seems that there is abnormal processing of pro-opiomelanocortin which may result in the release of ACTH with a higher than normal molecular weight termed ‘big ACTH’.
Gonadotrophin-releasing hormone and the gonadotrophins luteinising hormone and follicle-stimulating hormone
Gonadotrophin-releasing hormone (GnRH) is a decapeptide produced by the hypothalamus in pulses into the hypothalamic–hypophyseal portal circulation. GnRH stimulates the release of the gonadotrophins, luteinising hormone (LH) and follicle-stimulating hormone (FSH) from the gonadotroph. LH and FSH are dimeric glycopeptides and are released in pulses of approximately 90 min. This pulsatile release is essential for the gonadotrophins to exert their physiological actions. The release of GnRH is modified by oestrogens, progesterone, androgens and inhibins; these relationships are complex and are discussed in Chapter 10.
Thyrotrophin-releasing hormone and thyroid-stimulating hormone
Hypothalamic thyrotrophin-realeasing hormone (TRH) is a tripeptide that controls the secretion of thyrotrophin (TSH) by the thyrotroph cells of the anterior pituitary. Free thyroid hormones inhibit the release of TRH and TSH, but thyroid hormones exert their main negative feedback effects directly on TSH secretion (Figure 8.3).
TSH is a glycoprotein composed of an α-subunit that is common to LH, FSH and hCG, and a β-subunit specific to TSH. The place of TSH measurements in the investigation of thyroid function is discussed in Chapter 8.
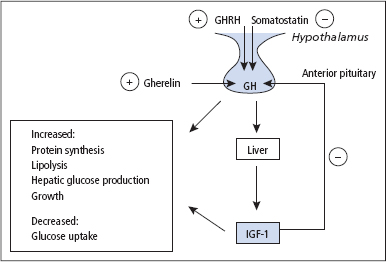
Growth hormone-releasing hormone and growth hormone
The release of GH by the somatotrophs in the pituitary is stimulated by hypothalamic GHRH, a 44 amino acid peptide, and inhibited by hypothalamic somatostatin, a 14 amino acid peptide. The peptide hormone ghrelin also stimulates the release of GH, acting in concert with GHRH to control both the timing and magnitude of GH release. Gherelin is a 28 amino acid peptide released predominantly from epithelial cells lining the fundus of the stomach. Gherelin also induces a feeling of hunger through action at the hypothalamus, and levels are very high in Prader-Willi syndrome patients who are obese and have voracious appetites. The release of GH is regulated to some extent by a negative feedback of IGF-1 on the pituitary to modify the action of GHRH and at the hypothalamus where IGF-1 together with GH stimulates the release of somatostatin, which in turn then downregulates GH release (Figure 7.2). GH release is suppressed by high doses of glucose, and this response is used in the investigation of suspected acromegaly and gigantism.
Figure 7.2 Factors which regulate the production and release of growth hormone (GH). In addition to the mechanisms shown, a regulatory effect on GH release is also achieved by insulin-like growth factor-1 (IGF-1) together with GH stimulating the release of somatostatin from the hypothalamus. In addition to GH promoting hepatic IGF-1 release, there is increasing evidence to suggest that GH may also promote the release of IGF-1 locally in target tissues, which then acts in a paracrine fashion. Gherelin also stimulates the release of GH, acting in concert with growth hormone-releasing hormone (GHRH) to control both the timing and magnitude of GH release. Gherelin is a 28 amino acid peptide released predominantly from epithelial cells lining the fundus of the stomach.
GH is a single polypeptide comprising 191 amino acids which circulates predominantly in a 22 kDa (75%) form, with the remainder circulating as a 20 kDa form or as glycosylated and sulphated derivatives. About half of the circulating GH is bound to a GH-binding protein, which is the cleaved extracellular domain of the GH receptor; the bound fraction has a longer half-life in plasma than free GH and as such the bound fraction has a prolonged bioactivity. GH is essential for normal growth, and originally it was thought that it acted mainly indirectly through stimulating the hepatic release of IGF-1 (also known as somatomedin C). In serum, IGF-1 circulates bound to six binding proteins (IGFBPs) with IGFBP3 being the most important. There is increasing evidence to suggest that GH may also promote the release of IGF-1 locally in target tissues, which in turn then acts in a paracrine fashion. The GH receptor is found in many tissues in the body, especially the liver. Stimulation of the receptor can give rise to activation of numerous signalling pathways which in turn allows GH to exhibit a wide variety of effects in different tissues.
Growth hormone deficiency
GH stimulates protein synthesis and growth, and also has metabolic effects that oppose the action of insulin, including increasing lipolysis and hepatic glucose production while decreasing tissue glucose uptake. It is now recognised that GH plays an important role in the adult as well as the child, and GH deficiency in the adult (most commonly caused by a pituitary tumour) is associated with significant morbidity and mortality. GH secretion occurs in pulses, mainly at night and lasting for approximately 1–2 h, giving rise to nocturnal peaks of approximately 12 μg/L. Only infrequent small pulses occur during the day, but exercise, stress, fasting, low blood [glucose] and ingestion of certain amino acids stimulate GH release, and this forms the basis of some of the stimulation tests used for the investigation of suspected GH deficiency. Measurement of basal [GH] is of little value since, during much of the day, levels are undetectable, but a low serum IGF-1 concentration is consistent with GH deficiency.
Prolactin
Prolactin is a single polypeptide (198 amino acids) secreted by the lactotrophs of the pituitary. The lactotrophs comprise 10% of the anterior pituitary cells in men but 30% in women. Prolactin acts via specific receptors which are widely distributed, but their main location is the mammary gland where their activation by prolactin stimulates lactation. Prolactin also has a role in the complex processes controlling gonadal function. High levels of prolactin can give rise to amenorrhoea, oligomenorrhoea, low libido and subfertility by impairing the pulsatile release of gonadotrophins.
Prolactin secretion is controlled by the inhibitory action of hypothalamic dopamine, but no hypothalamic stimulatory factor has been identified. There is a pulsatile release and diurnal rhythm of prolactin secretion, with the highest levels occurring during sleep and the lowest between 9 am and noon. Prolactin secretion increases in response to oestrogens, pregnancy, breastfeeding and stress. Certain drugs may also give rise to marked elevations in serum [prolactin].
A basal [prolactin] greater than 500 mU/L is regarded as being suspicious, but this should be confirmed on at least two or three occasions before follow-up investigations are initiated. There are many causes of hyperprolactinaemia other than pituitary disease (Table 7.2). Before collecting blood for measuring [prolactin], it is particularly important to enquire about any medication that the patient is taking since many drugs can give rise to hyperprolactinaemia (Table 7.2).
Table 7.2 Causes of hyperprolactinaemia
| Category of cause | Examples |
| Physiological | Pregnancy Breastfeeding Stress |
| Drugs | |
| Dopamine receptor- blocking agents | Phenothiazines Butyrophenones Tricyclic anti-depressants Metoclopramide |
| Dopamine-depleting drugs | Methyldopa Reserpine |
| Histamine receptor agonists | Cimetidine |
| Monoamine oxidase inhibitors | Phenelzine |
| Serotonin reuptake inhibitors | Paroxitine |
| Pathological | Prolactinoma Other pituitary tumours Idiopathic Chronic renal failure Primary hypothyroidism Macroprolactinaemia* |
* Most studies suggest that macroprolactinaemia is not associated with a clinical disorder unless monomeric prolactin is increased.
Prolactin measurements should be performed in the investigation of amenorrhoea, oligomenorrhoea and subfertility, whether or not there is galactorrhoea (p. 159). It should also be measured in any patient with spontaneous inappropriate lactation. A significant proportion of subfertile female patients have hyperprolactinaemia, and may respond to treatment with dopamine agonists such as cabergoline and bromocriptine.
Macroprolactin is formed when normal (monomeric) prolactin combines with autoantibodies in a patient’s serum to produce a prolactin–IgG complex (molecular weight 170 kDa), which is thought to be biologically inactive. The long half-life of the complex results in an elevated [total prolactin] being found in the serum. The clinical importance of macroprolactinaemia is controversial, with a few studies reporting clinical features typically associated with hyperprolactinaemia; however, most studies suggest that there is no associated clinical disorder. In view of this controversy, it is advisable to seek an endocrine opinion if macroprolactinaemia is found in symptomatic patients. All laboratories should assess whether a persistently elevated [total prolactin] is caused by macroprolactin. If macroprolactin is detected, the laboratory should estimate and report the concentration of normal (monomeric) prolactin that is present in the sample.
Anterior pituitary disease and its investigation
Causes
A wide range of conditions can affect the anterior pituitary and result in hypopituitarism either directly or through an effect on the hypothalamus (Table 7.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


