Regulation of adrenal steroid hormone synthesis and secretion
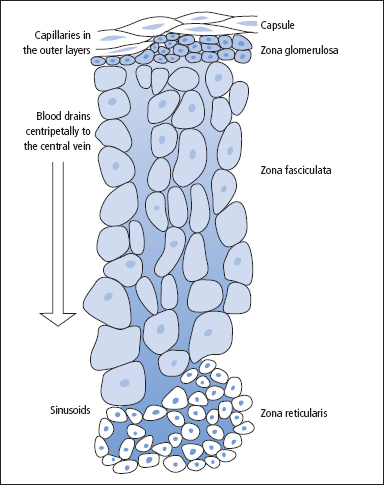
Three zones can be recognised in the adrenal cortex (Figure 9.1). The outermost zona glomerulosa is the site of synthesis of aldosterone, the principal mineralocorticoid. The deeper layers of the cortex, the zona fasciculata and zona reticularis, synthesise glucocorticoids, of which cortisol is the most important in man. Sex steroid production also occurs in the adrenal cortex, mainly in the zona reticularis and to some extent in the zona fasciculata.
Glucocorticoid secretion
Glucocorticoids have widespread metabolic effects on carbohydrate, fat and protein metabolism. In the liver, cortisol stimulates gluconeogenesis, amino acid uptake and degradation, and ketogenesis. Lipolysis is increased in adipose tissue, and proteolysis and amino acid release promoted in muscle. Glucocorticoids are also involved to some extent in regulating sodium and water homeostasis and the inflammatory and stress responses. In the circulation, glucocorticoids are mainly protein bound (~90%), chiefly to CBG (cortisol-binding globulin or transcortin). Plasma [CBG] is increased in pregnancy and with oestrogen treatment (e.g. oral contraceptives). It is decreased in hypoproteinaemic states (e.g. nephrotic syndrome). Changes in plasma [cortisol] occur in parallel to changes in [CBG]. The biologically active fraction of cortisol in plasma is the free (unbound) component, though usually the total (i.e. bound plus free) concentration of cortisol is measured for diagnostic purposes.
Figure 9.1 The morphological zonation of the adrenal cortex, showing the three types of cells and their particular structural arrangement.
ACTH is the main stimulus to cortisol secretion. Three factors regulate ACTH (and therefore cortisol) secretion:
Aldosterone secretion
The principal physiological function of aldosterone is to conserve Na+, mainly by facilitating Na+ reabsorption and reciprocal K+ or H+ secretion in the distal renal tubule and in other epithelial cells. Although its rate of production is less than 1% of the rate of cortisol production, aldosterone is a major regulator of water and electrolyte balance, as well as blood pressure.
The renin–angiotensin system is the most important system controlling aldosterone secretion (Figure 9.4). Renin is a proteolytic enzyme produced by the juxtaglomerular apparatus of the kidney and released into the circulation in response to a fall in circulating blood volume or renal perfusion pressure, and by loss of Na+. Renin then acts on angiotensinogen (a 248 amino acid peptide produced by the liver) in plasma to produce angiotensin I (AI), a decapeptide which is then converted by ACE in the lung to the octapeptide angiotensin II (AII). AI and particularly AII stimulate aldosterone production in the adrenal glomerulosa. Measurements of both aldosterone and renin are often helpful to establish whether aldosterone production is autonomous. Under normal circumstances, plasma [aldosterone] varies with posture, and measurement of [aldosterone] in the supine and erect position is also helpful in elucidating the cause of hyperaldosteronism (p. 145). An increase in plasma [K+] also stimulates aldosterone, while ACTH is relatively unimportant, except possibly in stress conditions and in congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (p. 299). No specific aldosterone-binding protein has been demonstrated.
Figure 9.2 The hypothalamic–anterior pituitary–adrenal axis and the fate of cortisol following its release. CBG = cortisol-binding globulin.
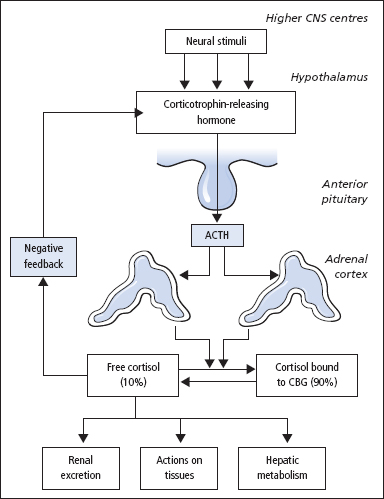
Figure 9.3 The diurnal rhythm of cortisol secretion; the shaded area represents values that lie within the reference range. There is a similar rhythm for the secretion of ACTH by the anterior pituitary. Patients with Cushing’s syndrome lose this diurnal variation.
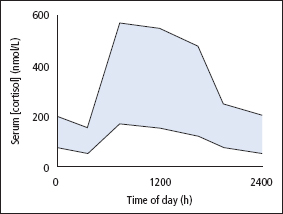
Figure 9.4 The renin–angiotensin system. Renin is released from the renal juxtaglomerular cells (JC) in response to hypotension, low blood volume or sodium depletion. Renin catalyses the conversion of angiotensinogen in plasma to angiotensin I. During passage through the lung, angiotensin-converting enzyme (ACE) catalyses the production of angiotensin II from angiotensin I. The angiotensin II stimulates release of aldosterone from the adrenal glomerulosa and the mineralocorticoid then promotes reabsorption of sodium in the distal tubules of the kidney.
Androgen secretion
The zona reticularis and to some extent the zona fasciculata produce the androgens androstenedione, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulphate (DHEAS). The measurements of these androgens are important in the investigation of hirsutism, virilisation (p. 162) and CAH (p. 298).
The pathways for the production of the various adrenal steroids are shown in Figure 9.5
Investigation of suspected adrenocortical hyperfunction
Hyperfunction of the adrenal cortex can lead to overproduction of cortisol (Cushing’s syndrome) or aldosterone (Conn’s syndrome).
Cushing’s syndrome
This can be ACTH dependent or ACTH independent (Figure 9.6; Table 9.1). If iatrogenic causes are excluded (e.g. use of hydrocortisone, prednisolone or dexamethasone), then the condition is caused by tumours that release either ACTH or cortisol. Approximately 70% of cases are due to a pituitary adenoma secreting ACTH (this is known as Cushing’s disease). Ectopic ACTH secretion (often from a small-cell carcinoma of the bronchus or a carcinoid tumour) is the cause of approximately 10% of the cases. Glucocorticoid-secreting adrenal adenoma or carcinoma are each responsible for about 10% of the cases. Most ACTH-dependent forms of Cushing’s syndrome lead to diffuse bilateral adrenocortical hyperplasia, but about 10–15% of patients with ACTH-driven Cushing’s syndrome demonstrate a macronodular hyperplasia.
While Cushing’s syndrome is uncommon, many of the clinical features of the disease such as hypertension, obesity, menstrual irregularities, depression, glucose intolerance or diabetes are commonly seen in general practice. It is therefore essential that patients with suspected Cushing’s syndrome should be investigated according to a logical scheme that involves the use of simple screening tests to establish a likely diagnosis of Cushing’s syndrome before the more involved investigations are carried out to establish the cause.
Some patients have ‘cyclical Cushing’s syndrome’ in which there is intermittent and cyclical abnormal secretion of ACTH. This may be reflected by a history of variable and intermittent depression with anxiety, or variability in the severity of the symptoms and signs characteristic of Cushing’s syndrome. Such patients may require tests to be performed on a number of occasions to make the diagnosis; salivary cortisol measurements provide a convenient means of providing this long-term assessment.
Figure 9.5 Steroid biosynthetic pathways in the adrenal cortex. Enzymes are shown in the boxes, and major steroid products are shown in bold. The conversion of corticosterone to aldosterone is restricted to the zona glomerulosa.
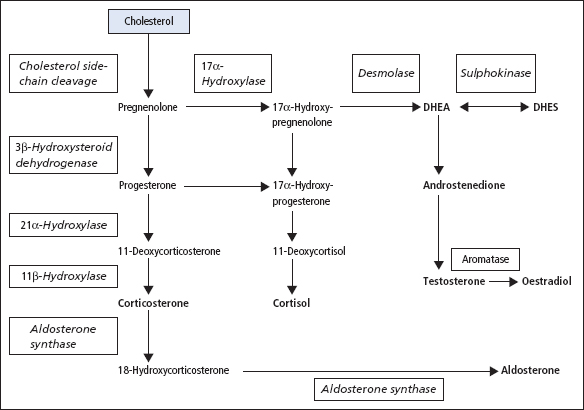
Figure 9.6 Pathological causes of Cushing’s syndrome. Cushing’s disease is caused by a pituitary adenoma autonomously secreting ACTH. The production of ACTH from the pituitary is suppressed (dotted arrow) when Cushing’s syndrome is caused by an ectopic ACTH-secreting tumour or an adrenal adenoma autonomously secreting cortisol.
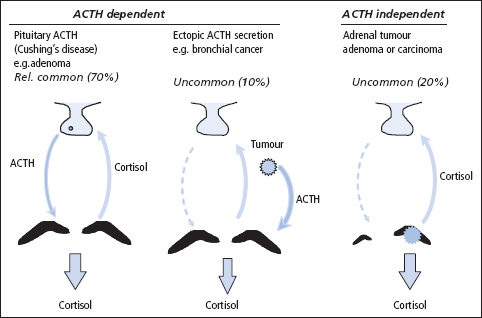
Table 9.1 Causes of adrenocortical hyperfunction
| ACTH dependent |
| Pituitary (Cushing’s disease) |
| Ectopic |
| Ectopic CRH or related peptides |
| ACTH therapy |
| ACTH independent |
| Adrenal adenoma |
| Adrenal carcinoma |
| Glucocorticoid therapy |
| Micronodular hyperplasia (partially ACTH dependent) |
A suggested plan of investigation is shown in Figure 9.7. Table 9.2 gives a summary of the commonly observed (but not invariable) findings in these hormonal tests.
Tests used to establish if a clinical diagnosis of Cushing’s syndrome is likely
Initial screening tests
Low-dose dexamethasone suppression test
The best initial screening test for adrenocortical hyperfunction is to perform, as an outpatient, a low-dose/overnight dexamethasone suppression test. Under normal circumstances, dexamethasone suppresses the secretion of CRH (negative feedback – Figure 9.2); ACTH and cortisol levels thus fall to below 50 nmol/L.
In the overnight suppression test, the patient takes dexamethasone (1 mg) at 11–12 pm the night before attending the clinic. Serum [cortisol] is measured on a blood specimen taken the following morning at 8–9 am. A cortisol concentration in serum of less than 50 nmol/L in this morning sample effectively excludes Cushing’s syndrome. This test performed using a 1 mg dose of dexamethasone has a false-positive rate of 12.5% with a false-negative rate of less than 2%.
The 48-h suppression test is superior, but less convenient than the overnight test. Dexamethasone (0.5 mg) is taken every 6 h, beginning at 9 am on the first day, and serum [cortisol] is measured 48h later (interpretation as for the overnight test). The true-positive rate is reported to be better than 97%, with a false-negative rate of less than 1% for the 48-h test.
Drugs that induce hepatic microsomal enzymes (e.g. phenytoin, phenobarbitone) may increase dexamethasone metabolism, with premature lowering of its blood level below that required to achieve suppression of CRH secretion (false-negative result).
Urinary free cortisol (UFC)
Cortisol undergoes hepatic metabolism with the production of a number of metabolically inactive compounds that are excreted in the urine mainly as conjugated metabolites (e.g. glucuronides). A small amount of cortisol is excreted unchanged in the urine. Urinary cortisol excretion is related to the biologically active plasma [free cortisol] during the period of urine collection.
The measurement of UFC excretion in a 24-h collection is an acceptable screening test, but it suffers from the disadvantage that an incomplete collection of urine may lead to a false-negative result. Cushing’s syndrome is excluded if the cortisol excretion is less than 250 nmol/24 h. Even if a complete urine collection is obtained, the test has a false-negative rate of 8–15%.
Interpretation of screening tests
The screening tests usually serve to distinguish simple non-endocrine obesity from obesity due to Cushing’s syndrome. Abnormal results may, however, be obtained in depressed or extremely anxious patients, in the presence of severe intercurrent illness or in alcoholism (pseudo-Cushing syndrome). Further tests (as an inpatient) may be required to rule out pseudo-Cushing syndrome and help determine the specific cause of the adrenocortical hyperfunction (Figure 9.7).
Confirmatory tests
Diurnal rhythm of plasma cortisol
In normal subjects, plasma cortisol is highest in the morning and lowest around midnight (Figure 9.3). Patients with Cushing’s syndrome lose this rhythm such that in most patients, the 8 am plasma [cortisol] is normal but the 10 pm plasma [cortisol] is raised. Patients need to be hospitalised for at least 48h prior to stress-free venepuncture (e.g. in-dwelling catheter) to obtain meaningful results. It is also convenient to collect a series of 24-h urine samples for urine free cortisol measurement.
Figure 9.7 Algorithm for the investigation of suspected Cushing’s syndrome and elucidation of its cause. CRH = corticotrophin-releasing hormone; CT = computed tomography; DXM = dexamethasone; MRI = magnetic resonance imaging. Some investigators may prefer to perform the CRH test rather than the insulin tolerance test.
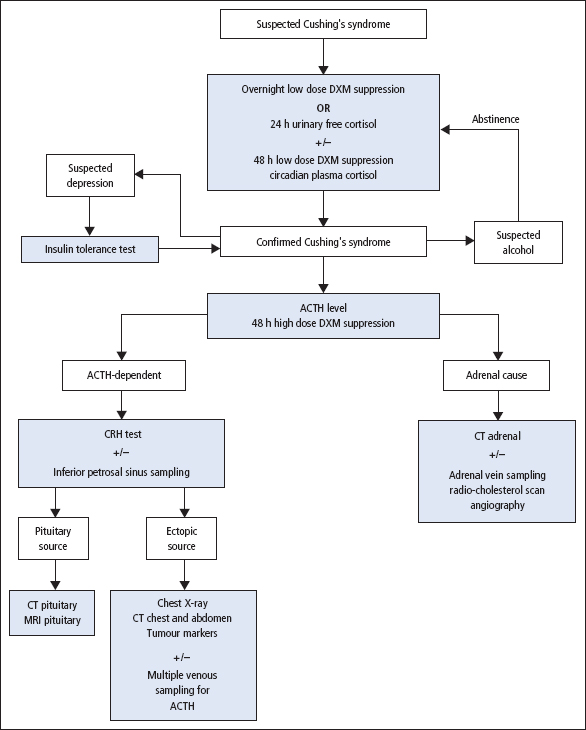
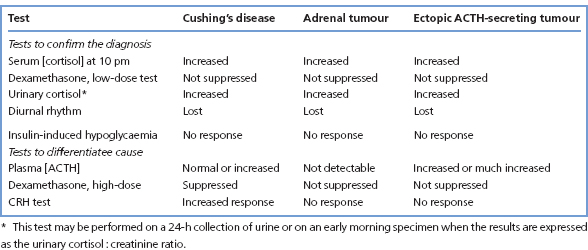
Table 9.2 Results of hormonal tests in patients with adrenal hyperfunction
Patients with pseudo-Cushing syndrome may also show abnormal diurnal variation in cortisol; however, in response to a hypoglycaemic stimulus, the pseudo-Cushing patient will increase secretion of CRH, ACTH and cortisol; in true Cushing’s syndrome, there is little or no response (see next section).
In alcoholism, other clues (e.g. raised mean cell volume (MCV), abnormal liver function tests) may be helpful; abstinence will lead to normalisation of the HPA axis.
Insulin hypoglycaemia test
This tests the integrity of the HPA axis, but is only occasionally necessary to allow true Cushing’s syndrome to be distinguished from depressive illness. The test is contraindicated in patients with epilepsy or heart disease.
Insulin is administered intravenously (typically 0.15 U/kg) to lower blood glucose to 2.2 mmol/L or less while monitoring serum [cortisol]. Samples for simultaneous measurement of glucose and cortisol are taken basally (before insulin) and at 30, 45, 60 and 90 min after IV insulin injection. The test requires close supervision, with glucose available for immediate injection if symptoms of severe hypoglycaemia develop. Failure to achieve a glucose level of 2.2 mmol/L invalidates the test, and repetition, with insulin incremented in steps of 0.05 U/kg, may be necessary.
Interpretation of results
Serum [cortisol] normally reaches its maximum at 60 or 90 min, the level reached being at least 500 nmol/L with an increment above the basal (pre-insulin) level of at least 145 nmol/L. Patients with Cushing’s syndrome, whatever the cause, show little or no increase in serum [cortisol], despite the production of an adequate degree of hypoglycaemia.
Determining the cause of Cushing’s syndrome
Once the diagnosis is established, other investigations may help to determine the cause (Table 9.2); difficulties sometimes arise in distinguishing between ectopic ACTH production and Cushing’s disease. Biochemical results need to be considered together with the findings from other methods of investigation, particularly radiological, for example MRI and CT scanning.
Plasma [ACTH]
ACTH is unstable and samples must be collected into EDTA with the plasma being separated and frozen within 30 min of collection. Temporary, often large, increases in these hormones may be observed as a response to emotional stress. Plasma [ACTH] should be measured on blood specimens collected both in the morning and in the evening (e.g. at 8 am and 10 pm). If ACTH is undetectable, this is diagnostic of a functional adrenal tumour, and should be confirmed by an abdominal CT or MRI scan to detect an adrenal mass. Rare types of nodular adrenocortical disease (also ACTH independent) are also described.
If the patient has Cushing’s disease (pituitary-dependent Cushing’s syndrome), ACTH will be present in plasma in normal or increased amounts, particularly in the evening specimen. Results for plasma [ACTH] overlap considerably for patients with Cushing’s disease and ectopic ACTH secretion. However, a very high plasma [ACTH] points to an ectopic (‘non-endocrine’) origin.
The following additional tests are used to distinguish Cushing’s disease from ectopic ACTH secretion.
High-dose dexamethasone suppression test
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


