Iron metabolism
The adult human possesses about 70 mmol (4 g) of iron. Iron balance is regulated by alterations in the intestinal absorption of iron. There is only a limited capacity to increase or decrease the rate of loss of iron.
Dietary iron and iron absorption
The normal intake of iron is about 0.2–0.4 mmol/ day (10–20 mg/day). Good sources are liver, fish and meat. Normally, about 5–10% of dietary iron is absorbed by an active transport process. Most absorption occurs in the duodenum. The rate of absorption is controlled by physiological and dietary factors:
- State of iron stores in the body Absorption is increased in iron deficiency and decreased when there is iron overload. The mechanism is unclear.
- Rate of erythropoiesis When this rate is increased, absorption may be increased even though the iron stores are adequate or overloaded.
- Contents of diet Substances that form soluble complexes with iron (e.g. ascorbic acid) facilitate absorption. Substances that form insoluble complexes (e.g. phytate) inhibit absorption.
- The chemical state of the iron Iron in the diet does not usually become available for absorption unless released during digestion. This depends, at least partly, on gastric acid production; Fe2+ is more readily absorbed than Fe3+, and the presence of H+ helps to keep iron in the Fe2+ form. Iron in haem (in meat products) can be absorbed while still contained in the haem molecule.
Iron transport, storage and utilisation
After being taken up by the intestinal mucosa, iron is either (1) incorporated into ferritin and retained by the mucosal cells, or (2) transported across the mucosal cells directly to the plasma, where it is carried mainly combined with transferrin (Figure 17.2). Iron retained by mucosal cells is lost from the body when the cells are sloughed. Mucosal cell retention is influenced by the body’s iron status, being reduced in iron depletion and increased in states of iron overload.
Figure 17.1 Schematic diagram illustrating the formation of haem, its incorporation into haem proteins and subsequent metabolism to bilirubin.
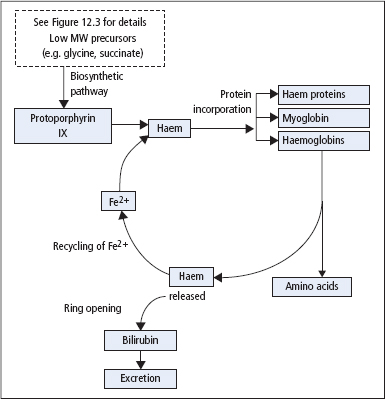
Figure 17.2 Summary of the absorption, transport and utilisation of iron. Total body iron stores (g) for the main iron-containing proteins as shown on the right side of the figure.
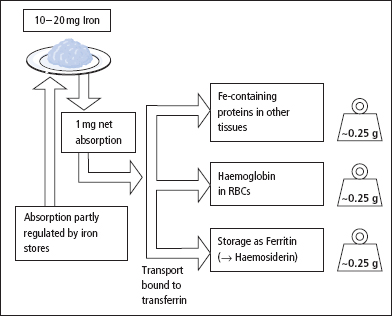
The total iron circulating bound to transferrin is normally about 50–70 μmol (3–4 mg). Iron in plasma is taken up by cells and either incorporated into haem or stored as ferritin (or haemosiderin, probably formed by the condensation of several molecules of ferritin). Iron released by the breakdown of Hb, at the end of the erythrocyte’s life, is normally efficiently conserved and later reused.
Iron excretion and sources of loss
Iron excreted in the faeces is principally exogenous, that is, dietary iron that has not been absorbed by the mucosal cells and transported into the circulation. In males, there is an average loss of endogenous iron of about 20 μmol/day (1 mg/day) in cells desquamated from the skin and the intestinal mucosa. Females may have additional losses due to menstruation or pregnancy. Urine contains negligible amounts of iron.
Laboratory assessment of iron status
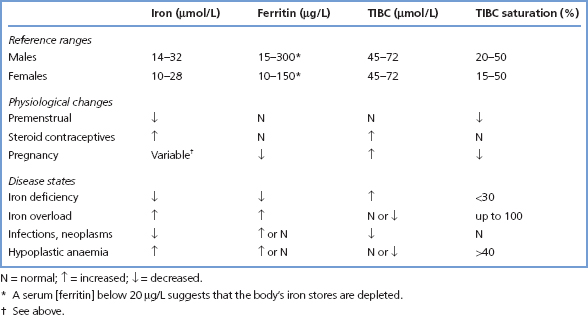
This is necessary in the investigation of iron deficiency states and iron overload. The following tests are used (for reference ranges, see Table 17.1).
Serum iron
This is of limited diagnostic value, since levels fluctuate widely in health. Much of this variation appears to be random, but some specific causes can be recognised:
Measurements of serum [iron] do not provide an adequate index of iron status. Although plasma [iron] is low in iron deficiency and is raised in iron overload, these changes occur relatively late when iron stores have already become either completely depleted or seriously overloaded. In addition, serum [iron] also alters in conditions not associated with changes in iron stores. Acute infections or trauma precipitate a rapid fall in serum [iron]. Chronic inflammatory disorders (e.g. rheumatoid arthritis) and malignant diseases are also associated with low levels.
Serum [iron] determination is only required for diagnostic purposes for a few conditions, for example in suspected cases of acute iron poisoning and in the assessment of individuals with an increased risk of haemochromatosis.
Table 17.1 Reference ranges for iron status
Serum ferritin
Serum [ferritin] is closely related to body iron stores, whether these are decreased, normal or increased, whereas serum [iron] becomes abnormal only in the presence of gross abnormalities of iron storage. A low, or low normal serum [ferritin] indicates the presence of depleted iron stores. However, since ferritin is an acute-phase reactant, levels may be increased in patients with iron deficiency and concurrent inflammation, malignancy or hepatic disease, although at concentrations greater than 100 μg/L iron deficiency is almost certainly not present.
Increased serum [ferritin] is found in iron overload, irrespective of the cause, and in many patients with liver disease or cancer. A normal serum [ferritin] virtually excludes untreated iron overload. Determination of serum [ferritin] currently provides the most useful measure of iron status widely available on a routine basis.
Serum transferrin, total iron-binding capacity and iron saturation
Normally, nearly all the iron-binding capacity in serum is due to transferrin, and about 40% of the binding sites on transferrin are occupied by iron. Transferrin has a much longer half-life than iron, and serum [transferrin] shows fewer short-term fluctuations. Transferrin levels fall in PEM, during the acute-phase response, and with infections, neoplastic disease and chronic liver disease. Synthesis increases in iron deficiency.
[Transferrin] can be measured not only directly, but also indirectly as the ability of serum protein (largely transferrin) to bind iron, the so-called TIBC of serum. The ratio of serum [iron] to [transferrin] (or TIBC) then determines the transferrin (or TIBC) saturation.
In iron-deficiency anaemia, the low serum [iron] is typically associated with an increase in transferrin concentration (and TIBC). This leads to a low saturation of transferrin (and TIBC) with iron. Conversely, in iron overload, serum [iron] is high and transferrin is normal or low, that is, a high percentage saturation of TIBC (Table 17.1). This effect is particularly marked in haemochromatosis, in which saturation of the TIBC usually rises above 60% fairly early in the disorder.
As with serum [iron], there is little place for determining serum [transferrin] or TIBC as a routine measure of iron status. However, in the detection of early or latent haemochromatosis, serum TIBC saturation should be measured. Also, in patients being treated with erythropoietin for the anaemia of chronic renal failure, the percentage saturation of TIBC provides a better index of available iron than serum [ferritin], and it is also a better guide to the need to give iron treatment.
The serum transferrin (or TIBC) is also helpful in determining the significance of very high serum [ferritin] in patients with disordered liver function of unknown cause, in whom the differential diagnosis may be between haemochromatosis and malignancy. A high serum [ferritin] in the absence of an increased percentage saturation of TIBC indicates that cancer is more likely to be the diagnosis.
Serum transferrin receptor
A circulating form of the transferrin receptor, lacking the cytoplasmic and transmembrane domains of the intact receptor, has been identified in serum. Its concentration rises in iron deficiency following the depletion of iron stores and, because levels are unaffected by inflammation, its measurement has the potential to provide an indication of iron status in patients with anaemia associated with chronic disease. Assays for serum transferrin receptor are not yet widely available.
Iron deficiency
Worldwide, this is the most common single nutrient deficiency. The main causes (Table 17.2) are deficient intake (including reduced bioavailability due to dietary fibre, phytates, etc.), impaired absorption (e.g. intestinal malabsorptive disease, abdominal surgery) and excessive loss (e.g. menstrual, GI bleeding).
Table 17.2 Causes of iron deficiency and excess
| Iron deficiency | Iron overload |
| Decreased intake | Excessive intake |
| Poor diet | Oversupplementation with iron tablets |
| Prolonged weaning (milk: poor iron source) | Repeated blood transfusions |
| Malabsorption | Iron utensils (especially with acid foodstuffs) |
| Increased requirements | Excessive absorption |
| (in the presence of inadequate intake) | Haemochromatosis |
| Adolescence | |
| Pregnancy | |
| Menstruating females | |
| Excessive iron losses | |
| Menorrhagia | |
| GI losses | |
| Genito-urinary losses | |
| Excessive blood donations |
In patients who develop iron deficiency,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


