Disorders of Haem Metabolism: Iron and the Porphyrias
Abnormalities of haem metabolism are important clinically. They include the porphyrias, which are rare, and disorders involving haem iron, such as iron deficiency, which are common in clinical practice.
HAEM METABOLISM
Most body tissues synthesize haem. In bone marrow it is incorporated into haemoglobin, an iron-containing pigment that carries oxygen from the lungs to tissues, and in muscle into myoglobin, which also binds oxygen. In other cells it is used for the synthesis of cytochromes and related compounds. The former are components of the electron transport chain, which is involved in oxidative phosphorylation. Quantitatively the liver is the major non-erythropoietic haemproducing organ. All haem pigments contain iron; the oxygen-carrying ability of the haem molecule depends on the presence of ferrous iron (Fe2+), the form normally present in both haemoglobin and oxyhaemoglobin.
Red blood cells are broken down by the reticuloendothelial system, predominantly in the spleen. Released haemoglobin is split into the peptide chain, globin, which enters the general protein pool, and haem. The haem ring is split, a process catalysed by haem oxidase, to form a linear molecule, biliverdin. Released iron is reused and biliverdin is reduced to lipid-soluble bilirubin. The metabolism of bilirubin is discussed in Chapter 17 in the context of jaundice.
Biosynthesis of haem and haemoglobin
The main steps in the synthesis of haem are outlined below and in Figure 21.1.
5-Aminolaevulinic acid (ALA) is formed by condensation of glycine and succinate. The reaction requires pyridoxal phosphate and is catalysed by ALA synthase. This is the rate-limiting step in the synthetic pathway and is regulated by feedback inhibition by haem.
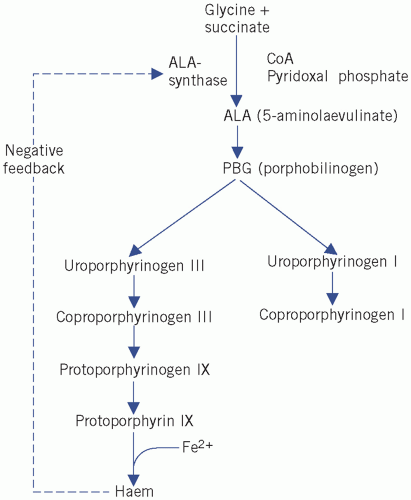
Figure 21.1 Biosynthesis pathways of haem. CoA, coenzyme A.
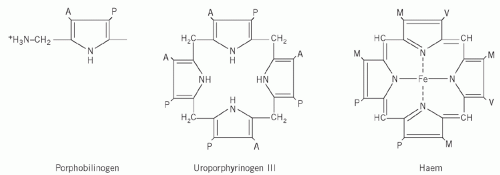
Four molecules of PBG combine to form a tetrapyrrole ring, uroporphyrinogen (Fig. 21.2). Two isomers are formed, I and III. The major pathway involves the III isomer.
Haem is formed by the successive production of coproporphyrinogen and protoporphyrin, followed by incorporation of Fe2+ into the centre of the ring.
Haemoglobin consists of four haem molecules, covalently linked to four (two pairs of) polypeptide chains.
Excretion of haem precursors
Excess intermediates on the haem pathway are excreted in either urine or faeces.
The porphyrin precursors ALA, PBG and uroporphyrinogen are water soluble and appear in the urine. They are colourless, but PBG may spontaneously oxidize to form uroporphyrin when exposed to air and light.
Porphyrinogens also oxidize spontaneously to the corresponding porphyrins, which are dark red and fluoresce in ultraviolet light. A urine specimen containing large amounts of porphyrinogens or their precursors will gradually darken if left standing. Protoporphyrin is excreted in bile and appears in the faeces, whereas coproporphyrin(ogen) may be excreted by either route.
Haemoglobin and related compounds
When oxygen is incorporated into haemoglobin to form oxyhaemoglobin, the spatial arrangement of the haem complexes is altered in such a way as to facilitate further oxygen uptake. Other compounds related to haemoglobin may sometimes be formed and some of these may hinder the oxygen-carrying capacity.
Carboxyhaemoglobin
Carboxyhaemoglobin is cherry red in colour and is formed when carbon monoxide binds to haemoglobin or displaces oxygen from oxyhaemoglobin; haemoglobin has a greater affinity for carbon monoxide than for oxygen. This occurs in carbon monoxide poisoning, which can be lethal. Once carbon monoxide is removed from inspired air, oxyhaemoglobin is reformed. Consciousness is not lost until the carboxy form has replaced about half the oxyhaemoglobin. (See oximetry and blood gases in Chapter 4.)
Methaemoglobin
Methaemoglobin is haemoglobin in which iron is in the ferric (Fe3+) form (haemin); therefore, it cannot carry oxygen. It is brown and is normally present in very low plasma concentrations; drugs such as sulphonamides or nitrites/nitrates may increase methaemoglobin. The symptoms of methaemoglobinaemia are due to hypoxia, which causes cyanosis and an increased respiratory rate and, if methaemoglobin is greater than 70 per cent of the total haemoglobin, can be fatal. Methemoglobin may cause a pulse oximeter to read about 85 per cent regardless of the actual amount of oxygen saturation. Methaemoglobinaemia is associated with glucose- 6-phosphate dehydrogenase (G6PD) deficiency (see Chapter 27).
Methaemalbumin
Methaemalbumin is also brown. It is formed when haem combines with plasma albumin in conditions such as severe intravascular haemolysis or acute haemorrhagic pancreatitis when haemoglobin has been converted to haemin in the abdominal cavity and absorbed. Methaemalbumin occurs when the haemoglobinbinding capacity of haptoglobin has been exceeded.
Sulphaemoglobin
Myoglobin
Myoglobin, a haem-protein complex, is normally present in muscle. Plasma concentrations may rise if skeletal muscle, e.g. rhabdomyolysis, or myocardial muscle cells, e.g. myocardial infarction, are damaged. Being of low molecular weight, it is rapidly cleared by the kidneys.
IRON METABOLISM
Distribution of iron in the body
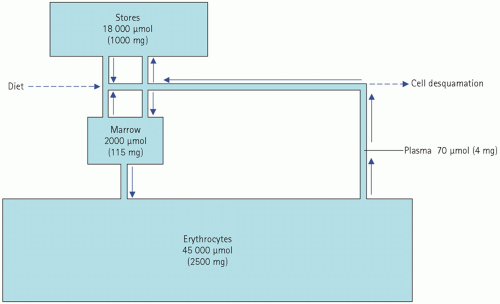
About 50-70 mmol (3-4 g) of iron are distributed among body compartments. There is considerable interchange of iron between stores and plasma. Free iron is toxic. In normal subjects it is all protein bound: in plasma it is bound to transferrin, in the storage pools to protein in ferritin and haemosiderin, and in erythrocytes it is incorporated into haemoglobin. About 70 per cent of the total iron is circulating, largely in erythrocyte haemoglobin. However, there are smaller amounts in muscle myoglobin and iron-containing enzymes and cytochromes.
Up to 25 per cent of the body iron is stored in the reticuloendothelial system, in the liver, spleen and bone marrow; bone marrow iron is drawn on for haemoglobin synthesis. Iron is stored in cells in the cytosol as ferritin or in the lyosomes as haemosiderin. Ferritin iron is more easily released from protein than that in haemosiderin. Haemosiderin can be seen by light microscopy in unstained tissue preparations. Ferritin and haemosiderin, but not haem iron, stain with potassium ferrocyanide (Prussian blue reaction), and this staining characteristic may be used to assess the size of iron stores.
Iron deficiency becomes haematologically evident only when no stainable iron is detectable in the reticuloendothelial cells in bone marrow films. Iron overload is likely when, because reticuloendothelial storage capacity is exceeded, stainable iron is demonstrable in parenchymal cells in liver biopsy specimens. This is the storage pool.
Only about 50-70 mmol (3-4 mg), or about 0.1 per cent of the total body iron, is circulating in plasma, all bound to transferrin; this fraction is measured in plasma iron assays. This is the transit pool. Iron can cross cell membranes only by active transport in the Fe2+ form; it is in this reduced state in both oxyhaemoglobin and ‘reduced’ haemoglobin. It is in the Fe3+ form in ferritin and haemosiderin and when bound to transferrin.
Iron absorption
Body iron content control depends on absorption by an active process in the duodenum: free Fe2+ via the divalent metal transporter 1 (DMT-1) and haem-bound iron via the haem carrier protein 1. Within the enterocyte, some of the iron combines with the protein apoferritin to form ferritin. Free iron is exported from the enterocyte via ferroportin into the circulation where it binds with transferrin for transport and storage. Transferrin-bound iron then enters target cells via receptor-mediated endocytosis. Hepcidin is the main iron regulating protein, and inhibits ferroportin. Concentrations of hepcidin increase in iron overload and reduce in iron deficiency. The human haemochromatosis protein (HFE) regulates the binding of transferrin to the transferrin receptor as well as regulating hepcidin.
Normally, about 18 µmol (1 mg) of iron is absorbed each day and this just replaces loss. This amounts to about 10 per cent of the iron ingested in the diet, although the proportion depends to some extent on the type of food. Once in the body, iron is in a virtually closed system (Fig. 21.3).
Iron absorption seems to be influenced by any or all of the following factors:
oxygen tension in the intestinal cells,
marrow erythropoietic activity,
the size of the body iron stores.
Iron absorption is also increased in many non-iron deficiency anaemias.
Most normal women taking an adequate diet probably absorb slightly more iron than men and so replace their higher losses in menstrual blood and during pregnancy.
Iron requirements for growth during childhood and adolescence are similar to, or slightly higher than, those of menstruating women and can be met by increased absorption from a normal diet.
Iron excretion
This is poorly controlled, as loss from the body may depend on the ferritin iron content of cells lost by desquamation, mostly into the intestinal tract and from the skin. The total daily loss by these routes is about 18 µmol (1 mg). Urinary excretion is minimal, as circulating iron is protein bound and not water soluble.
Normal iron loss is so small, and normal iron stores are so large, that it would take about 3 years to become iron deficient on a completely iron-free diet. Of course, this period is much shorter if there is any blood loss, such as menstruation.
Iron transport in plasma
Iron is transported in the plasma in the ferric form, attached to the specific binding protein transferrin at a concentration of about 18 µmol/L. Transferrin is normally capable of binding about 54 µmol/L of iron and is therefore about one-third saturated. Transferrinbound iron is carried to stores and to bone marrow cells and in the latter some iron passes directly into developing erythrocytes to form haemoglobin.
Factors affecting plasma iron concentration
The plasma iron concentration is likely to be a poor index of the total body content because only a very small proportion is in this compartment; it has no function, except as a protein-bound transport fraction. Plasma iron concentration is very variable, even under physiological conditions.
Physiological factors
The causes of physiological changes in plasma iron concentrations are not well understood, but alterations can be very rapid and almost certainly represent shifts between plasma and stores, not changes in total body iron. The following factors are known to affect plasma concentrations within a population:
Sex and age differences Plasma iron concentrations, like those of haemoglobin and the erythrocyte count, are higher in men than in women, probably for hormonal reasons. The difference is first evident at puberty, before significant menstrual loss has occurred, and disappears at the menopause. Androgens tend to increase the plasma iron concentration and oestrogens to lower it.
Pregnancy and oral contraceptives In the first few weeks of pregnancy, the plasma iron may rise to concentrations similar to those found in men. A similar rise occurs in women taking some oral contraceptives.
Iron concentrations can vary within an individual by up to 100 per cent or more. This can be due to the following:
Random variations Day-to-day variations may be as much as three-fold and usually overshadow cyclical changes. They may be associated with physical or mental stress or diet, but usually no cause can be found.
Circadian (diurnal) rhythm The plasma iron concentration is higher in the morning than in the evening. If subjects are kept awake at night, this difference is less marked; it is reversed in night workers.
Monthly variations in women The plasma iron may reach very low concentrations just before or during the menstrual period. The reduction is probably due to hormonal factors rather than blood loss.
Pathological and clinical factors
Iron deficiency and iron overload usually cause low and high plasma iron concentrations respectively. Iron deficiency is associated with a hypochromic, microcytic anaemia and with reduced amounts of stainable bone marrow iron. Plasma ferritin concentrations are usually, but not always, low. Iron overload is associated with increased amounts of stainable iron in liver biopsy specimens, and plasma ferritin concentrations are high.
Plasma iron is of little value in the diagnosis of iron deficiency not least because other pathological factors may affect plasma iron concentrations including the following:
Any acute or chronic illness (even a bad cold) causing a fall in plasma iron concentration Chronic conditions such as malignancy, renal disease, rheumatoid arthritis and chronic infections are often associated with normochromic, normocytic anaemia. Iron stores and plasma ferritin concentrations are normal or even increased; the anaemia does not respond to iron therapy. Iron deficiency may be superimposed on the anaemia of chronic illness, especially if drugs are being taken that cause gastrointestinal bleeding; plasma ferritin concentrations are then variable. The finding of hypochromic erythrocytes is the most sensitive index of this complication. Low plasma iron concentrations occur whether or not there is any iron deficiency.
Disorders in which the marrow cannot use iron, either because it is hypoplastic or because some other essential erythropoietic factor, such as vitamin B12 or folate, is deficient; plasma iron concentrations are often high. Blood and marrow films may show a typical picture, but, for example in pyridoxineresponsive anaemia and in thalassaemia, the findings in the blood film may resemble those of iron deficiency; in the last two conditions the presence of stainable marrow iron stores excludes the diagnosis of iron deficiency.
Haemolytic anaemia The plasma iron concentration may be high during a haemolytic episode, as iron, liberated from the destroyed erythrocytes, enters the plasma; it is usually normal during the quiescent periods when the iron enters the reticuloendothelial system. Marrow iron stores and plasma ferritin concentrations are usually increased in chronic haemolytic conditions.
Acute liver disease Disruption of hepatocytes may release ferritin iron into the bloodstream and cause a transient rise in the plasma iron concentration. Cirrhosis may be associated with a similar finding, perhaps due to increased iron absorption and intake.
Transferrin and total iron-binding capacity
Plasma iron concentrations alone rarely give information about the state of iron stores. In rare situations in which doubt remains after haematological investigation, diagnostic precision may sometimes be improved by measuring both the plasma transferrin and the iron concentrations.
Plasma transferrin can be directly assayed or measured indirectly by adding an excess of inorganic iron to the plasma, any not bound to protein being removed, usually with an exchange resin. The concentration of iron remaining is assayed and the result expressed as the total iron-binding capacity (TIBC). Unsaturated iron-binding capacity (UIBC) is the TIBC minus plasma iron concentration. This is usually a valid approximation of the transferrin concentration. In rare circumstances, of which the most common is severe liver disease, plasma ferritin concentrations are high enough to bind significant amounts of iron, and the results of iron-binding capacity measurements are then misleading as an assessment of the transferrin concentration.
Physiological changes in the plasma transferrin concentration
The plasma transferrin concentration is less labile than that of iron. However, it rises:
after about the 28th week of pregnancy even if iron stores are normal,
in women taking some oral contraceptive preparations,
in any patient treated with estrogens.
Pathological changes in the plasma transferrin concentration
Plasma transferrin concentration and TIBC:
rise in iron deficiency and fall in iron overload,
fall in those chronic illnesses associated with low plasma iron concentrations,
may be unchanged in acute illness,
may be very low in the nephrotic syndrome, associated with a low plasma iron concentration, because the relatively low-molecular-weight transferrin is lost in the urine together with iron.
Thus the low plasma iron concentration of uncomplicated iron deficiency is associated with a high transferrin concentration and TIBC; that of noniron deficiency is associated with low concentrations although plasma ferritin is the preferred investigation for iron deficiency. If iron deficiency coexists with the anaemia of chronic illness, the opposing effects of the two conditions on the transferrin concentration make it difficult to interpret transferrin, as well as plasma iron, concentrations.
FERRITIN
Circulating ferritin is usually in equilibrium with that in stores. However, it is an ‘acute-phase’ protein and its synthesis is increased in many inflammatory conditions. The normal plasma ferritin concentration is about 100 µg/L. A plasma ferritin concentration below about 10 µg/L suggests iron deficiency. Results can be misleading if there is coexistent inflammatory disease as accelerated synthesis may lead to normal or even high plasma concentrations despite very low iron stores. In this situation, the results of plasma iron and transferrin assays are also difficult to interpret; haematological parameters remain the most reliable diagnostic indicators of iron deficiency, and possibly also assay of the soluble transferrin receptor.
High concentrations of plasma ferritin usually occur in significant iron overload, but may also be due to:
Table 21.1 Biochemical findings associated with plasma iron abnormalities | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
inflammatory conditions,
malignant disease,
liver disease,
haemolysis,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree