Normal Skin
The skin is the most accessible organ of the human body. Its most basic function is simply a protective one. As a barrier, the skin holds off desiccation and disease by keeping moisture in and pathogens out. Nevertheless, characterization of the skin as mere “plastic wrap” is a gross underestimation of the anatomic and physiologic complexity of this vital structure.
Unlike parenchymal organs, end-organ dysfunction or failure is not a prerequisite for the diagnosis of a skin disease, because all skin diseases can be observed clinically irrespective of their functional effects. Among the spectacular array of neoplastic, inflammatory, infectious, and genetic cutaneous disorders, some elicit only trivial aberrations in skin structure or function, whereas others lead to profound and morbid consequences.
The integumentary system consists of a layer of tissue, 1–4 mm thick, that covers all exposed surfaces of the body. The skin merges uninterruptedly with the structurally similar envelope of the mucous membranes, but skin is distinct from mucosa in that it contains adnexal structures such as the eccrine units that exude sweat and the folliculosebaceous units that produce hairs and oils. There is considerable variation in skin thickness and composition, depending on the requirements of a particular body site. For example, the thinnest skin overlies the eyelids, where delicacy and mobility are essential. The thickest skin is present on the upper trunk, where sturdiness exceeds mobility in importance. The surfaces of the palms and soles are characterized by a high density of eccrine sweat units, reflecting the importance of this region in regulation of temperature; an absence of hairs, which would interfere with sensation; and accentuation of the cornified layer (see later discussion), contributing to the tackiness needed to handle objects deftly. The size of the structures between sites can also vary greatly, best illustrated by the contrast between large terminal hair follicles found on the scalp, bearded areas, and genital skin and the small vellus hair follicles found at most other sites.
Using a light microscope, two important skin layers are easily identifiable: a stratified squamous epithelium, the epidermis; and a layer of connective tissue, the dermis. The subjacent adipose tissue is considered as a third layer by some and is referred to as the subcutis.
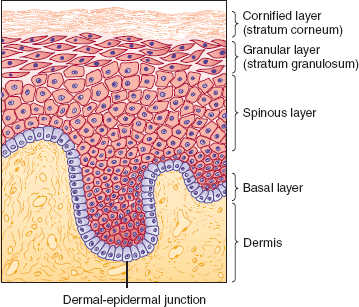
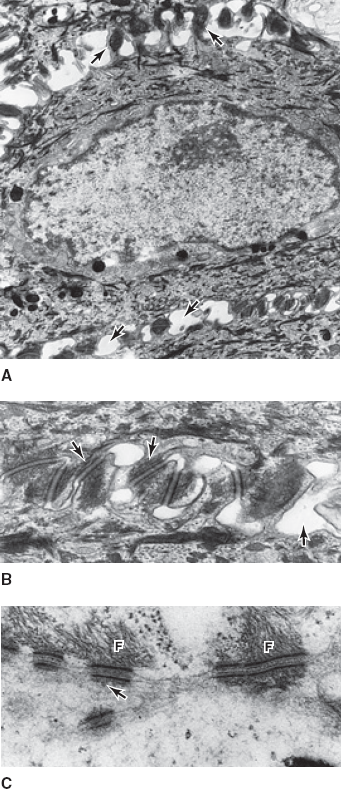
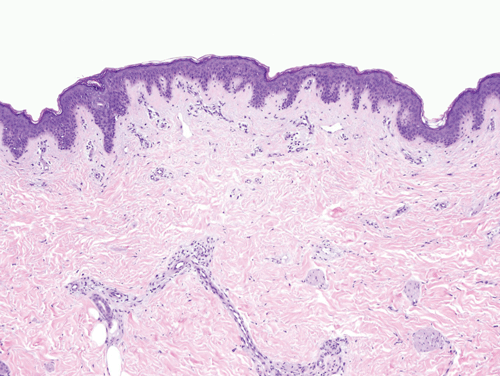
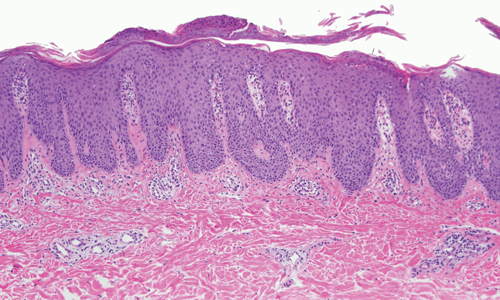
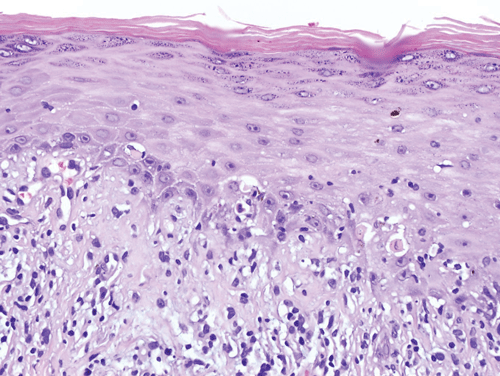
The epidermis consists of keratinocytes arrayed in four distinct substrata: the basal, spinous, granular, and cornified layers (Figure 8–1). Basal keratinocytes include the proliferative pool of keratinocytes. These cells divide, giving rise to progeny that are displaced toward the skin surface. As the keratinocytes move outwardly, they progressively flatten and accumulate keratin filaments within their cytoplasm. Individual keratinocytes are tightly bound together by intracellular junctions called desmosomes (Figure 8–2). The desmosomal junctions appear as delicate “spines” between cells in conventional microscopic sections and are most conspicuous in the epidermal spinous layer (Figure 8–3). Keratin filaments are linked intracellularly and are also attached to the desmosomes, forming a network that is vital to structural integrity.
Figure 8–1
Although the epidermis biologically displays a gradient of differentiation, four distinct layers are recognized on the basis of microscopic appearance. Cuboidal germinative keratinocytes serve as a foundation in the basal layer; cells with ample cytoplasm and prominent desmosomes constitute the spinous layer; cells with cytoplasmic granularity resulting from an accumulation of keratin complexes and other structural proteins are found in the granular layer; and anucleate, flattened keratinocytes comprise the tough, membrane-like cornified layer. (Redrawn, with permission, from Orkin M et al, eds. Dermatology. Originally published by Appleton & Lange. Copyright © 1991 by The McGraw-Hill Companies, Inc.)
Figure 8–2
In an ultrastructural view of a human keratinocyte (A), numerous desmosomes (B) appear as plaques that tightly bind two cell membranes together. With very high magnification (C), the attachment of cytoplasmic keratin filaments (F) to the desmosomes can be appreciated. (Reproduced, with permission, from Junqueira LO et al. Basic Histology, 10th ed. McGraw-Hill, 2003.)
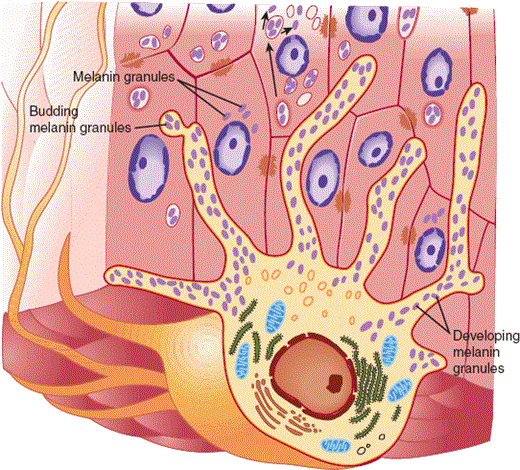
Melanocytes and Langerhans cells are dendritic cells that are intercalated among the keratinocytes of the epidermis. Melanocytes, which are positioned in the basal layer, synthesize a reddish-brown biochrome, melanin, and dispense it to numerous adjacent keratinocytes through their dendrites (Figure 8–4). This distribution system permits melanin to provide a dispersed screen against the potentially harmful ultraviolet rays of the sun. Langerhans cells share a similar arborized morphology but are positioned in the midspinous layer. Langerhans cells are bone marrow–derived antigen-presenting cells (see also Chapter 3).
Figure 8–4
The human melanocyte displays a branching morphology, and the dendrites of the cell maintain contact with 35–40 adjacent keratinocytes in a multicellular structure termed the epidermal melanin unit. The function of the unit is the effective dispersion of melanin pigment, packaged in organelles known as melanosomes, across a broad surface area. (Redrawn, with permission, from Junqueira LO et al. Basic Histology, 10th ed. McGraw-Hill, 2003.)
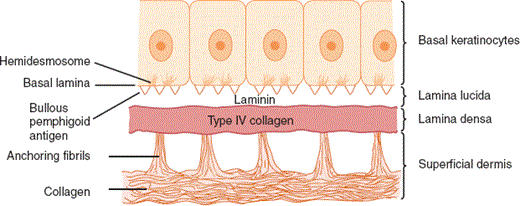
The epidermal-dermal junction, or basement membrane zone, is a structure that welds the epidermis to the dermis and contributes to the skin barrier. The juncture of the epidermis and dermis is arrayed in an undulating fashion to increase the surface area of binding between the two structures and to resist shearing forces. The downward projections of the epidermis are referred to as rete ridges, and the upward projections of the superficial dermis are called dermal papillae (Figure 8–5). Although the basement membrane comprises a thin eosinophilic (pink) band beneath the basal cells in conventional microscopic sections, it has a sophisticated multilayered structure that reaches from the hemidesmosomes of the basal keratinocytes to the collagen bundles of the superficial dermis (Figure 8–6). The lamina densa and lamina lucida are two of the layers of the basement membrane zone and are so named because of their electron-dense and electron-lucid appearance when viewed ultrastructurally.
The dermis consists of a connective tissue gel composed largely of proteins and mucopolysaccharides (so-called ground substance). This matrix serves as the scaffolding that supports the complex neurovascular networks, which course through the skin, and also supports the eccrine (sweat gland) and follicular (hair) adnexal structures. The vast majority of the fibrous structural proteins of the dermis are composed of collagen types I and III, and a network of elastic microfibrils is also woven throughout the full dermal thickness. Fibrocytes, the synthetic units of the structural proteins, are ubiquitous, and there are also mast cells and dendritic immune cells arrayed throughout the dermis. A discussion of the fine structure of the dermis—the dermal vascular and neural networks and the adnexal structures—is beyond the scope of this chapter.
Overview of Skin Diseases
In the broadest and simplest sense, there are two types of skin diseases: growths and rashes. A skin growth is a cyst, a malformation, or a benign or malignant neoplasm, something that presents clinically as a bump on the skin. A rash is, with rare exception, a nonneoplastic skin disease; it is more precisely referred to as an inflammatory skin condition or a dermatitis. The pathophysiologic aspects of the huge number of described growths and rashes exceed the scope of this chapter, and our discussion will focus on nine prototypical rashes.
Physicians interested in the skin learned decades ago that the accurate diagnosis and classification of the many patterns of dermatitis were dependent on a standardized nomenclature for the description and documentation of rashes. When used in association with a few well-chosen adjectives, the terms used to describe the prototypical types of inflammatory skin lesions (so-called primary lesions) permit vivid description of a rash. To illustrate the importance of terminology, imagine trying to describe a patient’s condition over the telephone to another physician. Talking about a red raised rash may truthfully describe the eruption in some sense, but the mental image evoked could be any one of dozens of skin diseases. The only way to accurately characterize an eruption is by the use of precisely defined terms.
The most important types of primary lesions include macules and patches, papules and plaques, vesicles and bullae, pustules, and nodules. The terms macule and patch denote flat areas of discoloration without any discernible change in texture. Macules are 1 cm or less in diameter, whereas patches exceed 1 cm in size. Papules and plaques are elevated, palpable skin lesions in which the breadth of the lesion exceeds its thickness. A papule is small, 1 cm or less in diameter, whereas a plaque exceeds 1 cm in size. Vesicles and bullae are fluid-filled spaces within the skin. Vesicles are less than 1 cm in diameter, whereas bullae exceed 1 cm in size. A vesicle or bulla containing purulent fluid is known as a pustule. A nodule is a solid, rounded skin lesion in which diameter and thickness are roughly equal.
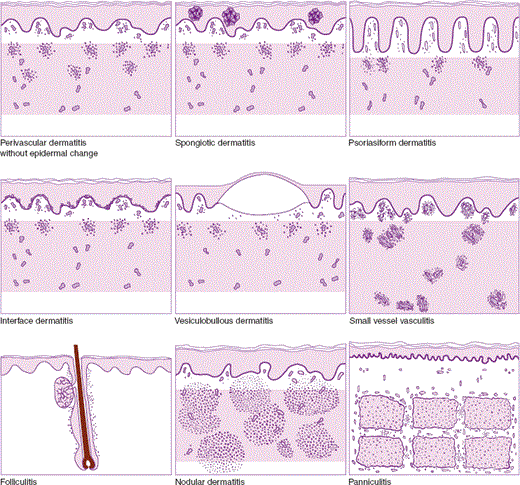
Different inflammatory processes involve different structures within the skin and display different microscopic patterns. Experience has shown that pattern analysis can serve as a useful means of diagnosis and classification. Pattern analysis is dependent on accurate recognition of the distribution of inflammation within the skin as well as recognition of the specific structures affected by the inflammatory reaction. There are nine distinct patterns of dermatitis (Table 8–1; Figure 8–7). Eight of these patterns and some of the diseases that produce them are discussed in detail next.
| Pattern | Description | Prototypes |
|---|---|---|
| Perivascular dermatitis | Perivascular inflammatory infiltrate without significant involvement of the epidermis | Urticaria (hives) |
| Spongiotic dermatitis | Inflammatory infiltrate associated with intercellular epidermal edema (spongiosis) | Allergic contact dermatitis (poison oak dermatitis) |
| Psoriasiform dermatitis | Inflammatory infiltrate associated with epidermal thickening as a result of elongation of rete ridges | Psoriasis |
| Interface dermatitis | Cytotoxic inflammatory reaction with prominent changes in the lower epidermis, characterized by vacuolization of keratinocytes | Erythema multiforme Lichen planus |
| Vesiculobullous dermatitis | Inflammatory reaction associated with intraepidermal or subepidermal cleavage | Bullous pemphigoid |
| Vasculitis | Inflammatory reaction focused on the walls of cutaneous vessels | Leukocytoclastic vasculitis |
| Folliculitis | Inflammatory reaction directed against folliculo-sebaceous units | Acne folliculitis |
| Nodular dermatitis | Inflammatory reaction with a nodular or diffuse dermal infiltrate in the absence of significant epidermal changes | Cutaneous sarcoidosis |
| Panniculitis | Inflammatory reaction involving the subcutaneous fat | Erythema nodosum |
Checkpoint
What are the two most basic barrier functions of skin?
How is skin distinct from mucosa? Why are these terms important?
What are the main primary lesions? Why are these terms important?
What are the main patterns of inflammatory skin disease?
What is the value of knowing the microscopic pattern of inflammation of a skin lesion? What additional information is needed for this information to be most useful?
Pathophysiology of Selected Skin Diseases
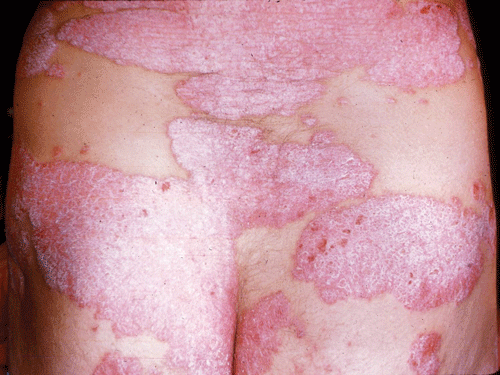
Psoriasis is a common chronic, persistent or relapsing, scaling skin condition. Individual lesions are distinctive in their classic form: sharply marginated and erythematous and surmounted by silvery scales (Figure 8–8). Most patients with psoriasis have a limited number of fixed plaques, but there is great variation in clinical presentation.
Psoriasis affects between 1% and 2% of individuals of both sexes in most ethnic groups. The most common age at onset is the third decade, but psoriasis can develop soon after birth, and psoriasis of new onset has been documented in a centenarian.
Several lines of evidence have established that genetic factors contribute to the development of psoriasis. There is a high rate of concordance for psoriasis in monozygotic twins and an increased incidence in relatives of affected individuals. The gene products of specific class I alleles of the major histocompatibility complex (MHC) are overexpressed in patients with psoriasis. Psoriasis is not merely a genetic disorder, however, because some susceptible individuals never develop characteristic lesions. In other predisposed individuals, a number of environmental factors, including infection, physical injury, stress, and drugs, can serve as triggers for the development of psoriasis (Table 8–2).
|
|
|
|
|
|
Psoriasis is the prototypical form of psoriasiform dermatitis, a pattern of inflammatory skin disease in which the epidermis is thickened as a result of elongation of rete ridges (Figures 8–7 and 8–9). In psoriatic lesions, epidermal thickening reflects excessive epidermopoiesis (epidermal proliferation). The increase in epidermopoiesis is reflected in shortening of the duration of the keratinocyte cell cycle and doubling of the proliferative cell population. Because of these alterations, lesional skin contains up to 30 times as many keratinocytes per unit area as normal skin. Evidence of excessive proliferation is also manifest microscopically as numerous intraepidermal mitotic figures.
During normal keratinocyte maturation, nuclei are eliminated as cells enter the cornified layer and condense to form a semipermeable envelope. In psoriasis, the truncation of the cell cycle leads to an accumulation of cells within the cornified layer with retained nuclei, a pattern known as parakeratosis. As parakeratotic cells accumulate, neutrophils migrate to the cornified layer. Histopathologically, the silvery scale of psoriatic plaques consists of a thick layer of parakeratotic keratinocytes with numerous intercalated neutrophils. At times, the number of neutrophils in the stratum corneum is so great that lesions assume a pustular appearance.
Psoriasis also induces endothelial cell hyperproliferation that yields pronounced dilation, tortuosity, and increased permeability of capillaries in the superficial dermis (Figure 8–10). The vascular alterations contribute to the bright erythema seen clinically. The capillary changes are most pronounced at the advancing margins of psoriatic plaques.
After years of research, a large number of immunologic abnormalities that involve both innate and adaptive immunity have been documented in psoriatic skin. Antigenic stimuli are thought to activate the innate immune response, leading to the production of cytokines, such as interferon, tumor necrosis factor (TNF), interleukin-23 (IL-23), and IL-12, by macrophages, dendritic cells, and neutrophils. This leads to attraction, activation, and differentiation of T cells. These T cells, most importantly T helper 1 and T helper 17 cells, produce cytokines that lead to epidermal hyperplasia, recruitment of inflammatory cells, and ultimately a positive feedback loop that perpetuates the pathologic process.
The cardinal features of the plaques of psoriasis include sharp margination, bright erythema, and nonconfluent whitish or silvery scales. Lesions can occur at any site, but the scalp, the extensor surfaces of the extremities, and the flexural surfaces are often involved. Psoriasis commonly affects the nail bed and matrix, yielding pitted or markedly thickened dystrophic nails. Mucosal surfaces are spared.
The only extracutaneous manifestation of psoriasis is psoriatic arthritis, a deforming, asymmetric, oligoarticular arthritis that can involve small or large joints. The distal interphalangeal joints of the fingers and toes are characteristically involved. Psoriatic arthritis is classified as one of the seronegative spondyloarthropathies, distinguishable from rheumatoid arthritis by a lack of circulating autoantibodies (so-called rheumatoid factors) or circulating immune complexes and by linkage with specific MHC class I alleles, including HLA-B27.
There are many variants of psoriasis, all of which are histopathologically similar but which differ greatly in clinical distribution (Table 8–3).
| Variant | Cutaneous Findings and Distribution | Other Features |
|---|---|---|
| Plaque-type psoriasis (psoriasis vulgaris) | Large stationary plaques with prominent scales, commonly involving the scalp and the extensor surfaces of the extremities | |
| Guttate psoriasis | Scaling papules or small plaques, usually 0.5–1.5 cm in diameter, scattered on the trunk and proximal extremities | Lesions often induced or exacerbated by streptococcal pharyngitis |
| Erythrodermic psoriasis | Generalized erythematous plaques involving the face, trunk, and extremities, with only slight scaling | |
| Pustular psoriasis, generalized | Generalized eruption of sterile pustules involving erythematous skin of the trunk and extremities, often with sparing of the face | Associated with fever; may occur in pregnancy |
| Pustular psoriasis, localized | Scaling erythematous plaques, studded with pustules, involving the palms, soles, and nails | |
| Inverse psoriasis | Slightly scaling, erythematous plaques involving the axillary and inguinal regions, with sparing of areas usually involved in plaque-type disease |
Checkpoint
What evidence supports a genetic role in the development of psoriasis? An environmental role?
Which cell types hyperproliferate in psoriasis?
What immunologic defects have been identified in psoriasis?
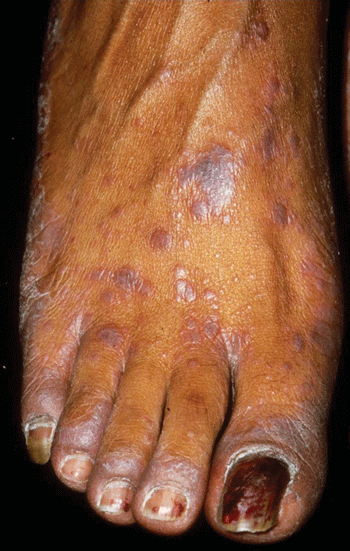
Lichen planus is a distinctive itchy eruption that usually presents with numerous small papules. Individual lesions have angulate borders, flat tops, and a violaceous hue, attributes that form the basis of their alliterative description as pruritic polygonal purple papules (Figure 8–11). The individual papules of lichen planus sometimes coalesce to form larger plaques. Minute whitish streaks, barely visible to the naked eye and known as Wickham striae, are characteristically found on the surfaces of lesions.
Lichen planus generally develops in adulthood and affects women slightly more commonly than men. Although the factors that trigger lichen planus remain obscure in many patients, it is clear that the rash represents a cell-mediated immune reaction that directly or indirectly damages basal keratinocytes of the epidermis. Observations that suggest a cell-mediated mechanism include the occurrence of lichen planus-like eruptions as a manifestation of graft-versus-host disease after bone marrow transplantation and the development of a lichen planus–like eruption in mice after injection of sensitized, autoreactive T cells. Although most lichen planus is idiopathic, drugs are one established cause of lichen planus or lichen planus–like reactions. Therapeutic gold and antimalarial agents are the medications most closely linked to the development of lichenoid eruptions, but a long list of other agents has accumulated (Table 8–4).
|
|
|
|
|
|
|
|
|
|
|
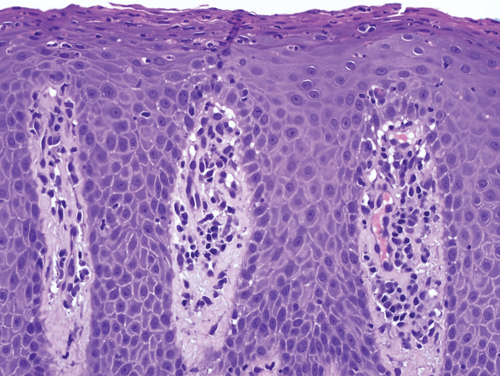
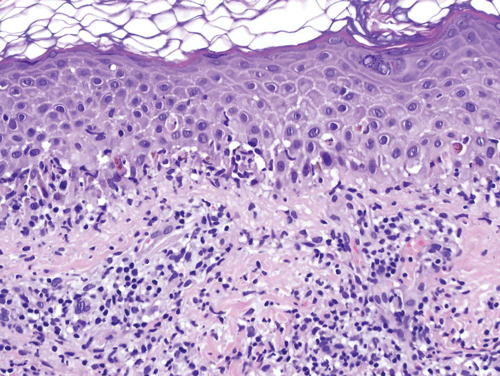
Lichen planus is a form of lichenoid interface dermatitis, a type of inflammatory skin disease in which a dense infiltrate of lymphocytes occupies the papillary dermis and the superficial dermis immediately subjacent to the epidermis, in association with vacuolization of the lower epidermis (Figure 8–12). The papillary dermal infiltrate is composed largely, if not entirely, of T lymphocytes. Some of the T cells are also found within the epidermis, where adjacent vacuolated, injured keratinocytes are found. Dense eosinophilic (pink) globules, known as colloid bodies, are also identifiable within the epidermis and the infiltrate (Figure 8–13). Colloid bodies represent condensed, anucleate keratinocytes that have succumbed to the inflammatory reaction. Although the keratinocytes bear the brunt of the lymphocyte attack, melanocytes may be coincidentally destroyed in the reaction as “innocent bystanders.” Free melanin pigment is released as melanocytes are damaged, and the pigment is phagocytosed by dermal macrophages known as melanophages.
In incipient lesions of lichen planus, CD4 helper T lymphocytes predominate, and some of the cells have been found in proximity to macrophages and Langerhans cells (see also Chapter 3). In contrast, CD8 cytotoxic T cells comprise the bulk of the infiltrate in mature lesions. This shift in infiltrating T-cell composition is thought to reflect the afferent and efferent aspects of lesional development. In the afferent phase, causative antigens are processed and presented to helper T cells, probably in the context of specific HLA determinants. The stimulated CD4 lymphocytes then elaborate specific cytokines that lead to the recruitment of cytotoxic lymphocytes. Cell-mediated cytotoxicity and cytokines such as interferon-γ and TNF are then thought to contribute to the vacuolization and necrosis of keratinocytes as a secondary event.
The clinical appearance of lichen planus lesions reflects several synchronous alterations in the skin. The dense array of lymphocytes in the superficial dermis yields the elevated, flat-topped appearance of each papule or plaque. The chronic inflammatory reaction induces accentuation of the cornified layer (hyperkeratosis) of the epidermis, which contributes to the superficial whitish coloration perceived as Wickham striae. Although the many melanophages that accumulate in the papillary dermis hold a brownish black pigment, the fact that the pigmented cells are embedded in a colloidal matrix such as the skin permits extensive scattering of light, a phenomenon known as the Tyndall effect. Thus, the human eye interprets a lesion of lichen planus as dusky or violaceous despite the fact that the pigment that serves as the basis for the coloration is melanin.
Lichen planus affects both skin and mucous membranes. Papules are generally distributed bilaterally and symmetrically. The sites most commonly involved include the flexor surfaces of the extremities, the genital skin, and the mucous membranes. Rarely, lichen planus may involve the mucosa of internal organs, such as the esophagus. Cutaneous lesions are virtually never seen on the palms, soles, or face.
In general, lichen planus variants can be grouped into three categories.
In these variants, typical individual papules of lichen planus are grouped in a distinctive larger pattern. In annular lichen planus, small lichenoid papules coalesce to form a larger ring. Linear and zosteriform patterns of lichen planus have also been observed. When lichen planus presents in an unusual configuration, it is prone to be underdiagnosed or misdiagnosed.
Although most lichen planus is widespread, at times papules are restricted to a specific body site, such as the mouth (oral lichen planus) or genitalia. Nearly 25% of all lichen planus patients have disease limited to the mucous membranes.
Some examples of lichen planus defy clinical recognition because the appearance of the individual lesions is atypical. Erosive, vesiculobullous, atrophic, and hypertrophic lesions can be seen. In erosive lichen planus, the interface reaction that is directed against the epidermis is so profound that the entire epidermis becomes necrotic and ulceration ensues. The closely related entity vesiculobullous lichen planus is also characterized by an intense interface reaction that yields necrosis of the epidermal junctional zone across a broad front. As a result of basal layer necrosis, the epidermis becomes detached from its dermal attachments and a blister develops. In atrophic lichen planus, the rate of destruction of keratinocytes by the lichenoid interface reaction exceeds the rate of epidermal regeneration, and the epidermis becomes attenuated as a result. In contrast, in hypertrophic lichen planus, the rate of epidermal regeneration triggered by the interface reaction exceeds the rate of destruction, and thick, verrucous, hyperkeratotic lesions develop. All of these variants are histopathologically similar with the exception of the foci of ulceration seen in erosive lichen planus.
Checkpoint
What skin cells are damaged by cell-mediated immune reactions in lichen planus?
Which drugs have been most commonly implicated in licheniform eruptions?
What synchronous alterations in the skin are reflected in the clinical appearance of lichen planus?
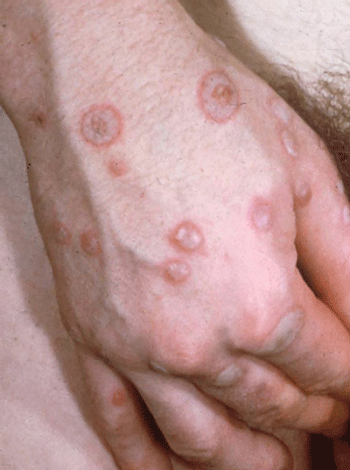
Erythema multiforme is an acute cutaneous eruption that presents with a wide spectrum of clinical severity. The eruption is commonly brief and self-limited, but repetitive or generalized attacks can be disabling or even life threatening. As the name implies, variation in lesional morphology can be seen, but most patients present with a monomorphous pattern in a given bout. The prototypical lesion is a red macule or thin papule that expands centrifugally and develops a dusky or necrotic center, creating a target-like pattern (Figure 8–14).
Erythema multiforme is an uncommon but distinctive skin disease that afflicts men and women in nearly equal numbers. The peak incidence is in the second to fourth decades of life, and onset during infancy or early childhood is a rarity. Like lichen planus, erythema multiforme represents a cell-mediated immune reaction that eventuates in necrosis of epidermal keratinocytes. Herpes simplex viral infection and reactions to medications have been established as the most common causes of erythema multiforme. Other known causes include Mycoplasma infection, contact dermatitis, drugs, and radiation.
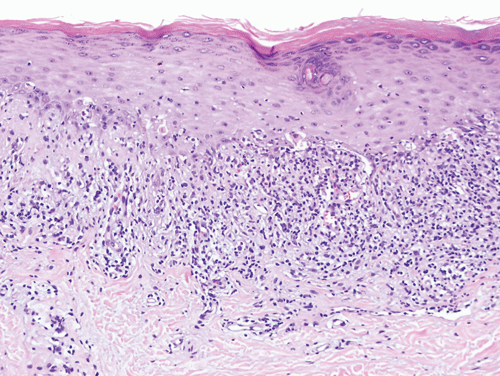
Erythema multiforme is a prototypical form of vacuolar interface dermatitis. In contrast to lichen planus, which typically presents with a dense obscuring lichenoid infiltrate within the superficial dermis, in erythema multiforme the inflammatory infiltrate is sparse. Thus, the vacuolated keratinocytes that are widely distributed within the epidermal basal layer are conspicuous in the face of a sparse infiltrate, and the damaged keratinocytes serve as the basis for the name of this pattern of inflammatory skin disease.
The dermal infiltrate in erythema multiforme is composed of a mixture of CD4 and CD8 T lymphocytes. CD8 cytotoxic cells are also found within the epidermis, in proximity to vacuolated and necrotic keratinocytes. Keratinocytes that are killed in the course of the inflammatory reaction become anucleate and are manifest microscopically as round, dense, eosinophilic bodies similar to the colloid bodies of lichen planus (Figure 8–15).
Although lichen planus and erythema multiforme are clinically, microscopically, and etiologically distinct, both appear to share a common pathogenetic pathway in which specific inciting agents recruit effector lymphocytes into the epidermis and papillary dermis. After this recruitment, keratinocytes are injured and killed by the combined negative influences of cytotoxicity and cytokines, such as interferon-γ and TNF.
Many cases of so-called erythema multiforme minor are triggered by herpes simplex viral infection. A relationship between erythema multiforme and herpetic infection had long been suspected based on the documentation of preceding herpes simplex lesions in patients with erythema multiforme. The relationship was strengthened after antiherpetic drug therapy, in the form of oral acyclovir, was shown to suppress the development of erythema multiforme lesions in some individuals. Molecular studies have substantiated the relationship by confirming the presence of herpes simplex DNA within skin from erythema multiforme lesions. Herpesvirus DNA is also demonstrable within peripheral blood lymphocytes and within lesional skin after resolution but not within nonlesional skin. These findings suggest that viral DNA is disseminated from the primary infection in the peripheral blood and becomes integrated into the skin at specific target sites. The herpetic genomic fragments then contribute to the development of a cytotoxic effector response in their chosen target tissue, the skin.
The target-like clinical appearance of many erythema multiforme lesions reflects zonal differences in the intensity of the inflammatory reaction and its deleterious effects. At the periphery of an erythema multiforme lesion, only sparse inflammation, slight edema, and subtle vacuolization of the epidermis are apparent in the outer erythematous halo. In contrast, the dusky “bull’s eye” often shows pronounced epidermal vacuolization, with areas of near-complete epidermal necrosis.
Erythema multiforme is generally limited to the skin and mucous membranes. The lesions develop rapidly in crops and are initially distributed on acral surfaces, although proximal spread to the trunk and face occurs not uncommonly. Mucosal erosions and ulcers are seen in roughly 25% of cases, and mucositis can be the sole presenting feature of the disease. Although erythema multiforme is an epithelial disorder, nonspecific constitutional symptoms such as malaise can also occur.
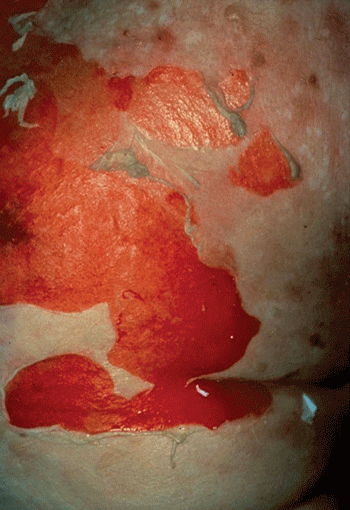
Although the spectrum of erythema multiforme exists as a continuum, a given patient is usually classified as having minor or major disease. The disorder is referred to as erythema multiforme minor when there are scattered lesions confined to the skin or when skin lesions are observed in association with limited mucosal involvement. A diagnosis of erythema multiforme major is based on the presence of prominent involvement of at least two of three mucosal sites: oral, anogenital, or conjunctival. Many examples of erythema multiforme major also display severe, widespread cutaneous involvement. Although Stevens-Johnson syndrome had classically been used to describe severe cases of erythema multiforme, consensus classification has separated Stevens-Johnson syndrome from erythema multiforme and added it to the spectrum of toxic epidermal necrolysis. These two entities, Steven-Johnson syndrome and toxic epidermal necrolysis, are now considered to represent variant dermatologic manifestations of severe idiosyncratic reactions. Most often the result of medications, these entities involve vast regions of skin and mucosal necrosis (Figure 8–16) with secondary vesiculation. Pathologically, the findings are similar to those of a severe burn in that the integrity of a patient’s skin fails, resulting in an increased risk of infectious and metabolic sequelae.
Figure 8–16
Toxic epidermal necrolysis. Generalized maculopapular erythema of the trunk and extremities is followed by extensive desquamation, as illustrated on this patient’s trunk, resulting from epidermal necrosis. Patients are often admitted to a burn unit for acute care. (Image used with permission from Dr. Timothy Berger.)
Checkpoint
What is the prototypical lesion in erythema multiforme?
In what ways is erythema multiforme similar to and different from lichen planus?
What are some complications of toxic epidermal necrolysis?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree