Diffuse and Nodular Hyperplasia
Paul W. Biddinger
GRAVES DISEASE (DIFFUSE TOXIC HYPERPLASIA)
Definition
Graves disease, also known as diffuse toxic hyperplasia or diffuse toxic goiter, is an autoimmune disorder characterized by excessive production of thyroid hormone and diffuse hyperplasia with enlargement of the thyroid. In addition to generalized manifestations of hyperthyroidism, Graves disease is associated with distinct extrathyroidal lesions including inflammation of the orbital tissues, known as Graves ophthalmopathy, and excessive accumulation of glycosaminoglycans in the skin. The latter most often occurs in the anterior region of the leg and is known as pretibial myxedema.
Historical Comments
The commonly used eponymous term Graves disease recognizes the Irish physician Robert Graves. In 1835, he published lectures describing several cases of cardiac palpitations associated with enlargement of the thyroid and, in one case, exophthalmos.1 Karl Adolph von Basedow published his description of the triad of goiter, palpitations, and exophthalmos in 1840, and his name is also linked eponymously to this disease, particularly in Europe.2 Caleb Parry predated Graves and von Basedow with his description of the signs and symptoms of hyperthyroidism in association with goiter in an 1825 posthumous publication.3 Several other apparent descriptions consistent with Graves disease preceded all of the above, some dating back into antiquity, and it seems quite probable that there were other reports now lost or very difficult to access.
Appreciation of thyroid hyperfunction as the principal explanation for clinical features evolved over time. Parry and Graves considered the disease to be primarily a disorder of the heart, whereas others considered it a disease of the nervous system. In 1884, Ludwig Rehn reported cure of toxic signs and symptoms with thyroidectomy, and in an 1886 publication, Paul Möbius gave support to thyroid hyperfunction as the prime abnormality.4,5 By the early 20th century, the disease was well established as a thyroid disorder.
Several important events occurred in the middle of the 20th century. Antithyroid drugs were developed and used in cases of Graves disease as was radioactive iodine.6,7 Adams and Purves identified long-acting thyroid stimulator (LATS) in 1956, and subsequent studies revealed that it was an immunoglobulin directed against thyroid-stimulating hormone receptor (TSHR).8,9 Building on these key pieces in the puzzle, our knowledge of Graves disease has grown considerably over the past half century.
Epidemiology
Graves disease is one of the most common autoimmune diseases with an estimated prevalence of approximately 0.4% to 1% in the US population.10,11,12 Prevalence and incidence data of various studies are difficult to compare because of differences in diagnostic criteria and the changes in analytic technology over time. Some studies do not differentiate Graves disease from other causes of hyperthyroidism such as toxic multinodular goiter and toxic adenoma. The annual incidence of Graves disease in iodine-sufficient regions usually falls in the range of 20 to 25 per 100,000.12 The disease can affect all ages but is rare before adolescence and has a peak incidence in the fourth to sixth decades.10,13,14 Women are three to eight times more commonly affected than men.10,11,13,14,15,16
Etiologic Factors
Both environmental and genetic risk factors play roles in the development of Graves disease.17,18 The precise etiology is unknown, but the disease appears to develop in the setting of genetic susceptibility combined with one or more environmental triggers that precipitate an autoimmune response. Female gender is a major risk factor, similar to most other autoimmune diseases. Explanations tend to involve immune modulation, but much remains uncertain.
Iodine supplementation of previously deficient populations is associated with an increased incidence of hyperthyroidism (Jod-Basedow phenomenon).19 Most cases are due to toxic multinodular goiter; however, subclinical cases of Graves disease may be unmasked when increased iodine intake permits increased thyroid hormone production. In a comparative study of genetically similar populations in regions with high- and low-dietary iodine, the annual incidence of Graves disease was greater in the region with high intake.20 However, the total annual incidence of thyrotoxicosis owing to all causes was higher in the region with low-dietary iodine. The relationship of iodine intake to Graves disease seems in large part an unmasking of the underlying autoimmune process, but a more direct role may exist. Iodine may be capable of follicular cell injury by production of autoreactive thyroid epitopes or generation of reactive metabolites.17,21
Stress is suspected as a significant etiologic factor. An increase in the incidence of Graves disease has been observed after stressful life events and in regions affected by war.17,22,23 Although attempts to link stress with Graves disease may be problematic in terms of methodology, stress is generally accepted as having significant effects on the immune system, and such effects may potentiate an autoimmune process.24
Women have about a 6-fold increase in risk of Graves disease during the first year postpartum.25 During pregnancy, many autoimmune disorders decrease in severity, apparently reflecting increased immune tolerance important for the health of the fetus. Inflammation and autoantibody titers typically decrease. Post partum, a rebound of immunologic activity including production of antibodies to thyroid stimulating hormone (TSH) receptors, can manifest as the initial presentation or relapse of Graves disease.26 Another possible reason pregnancy is a risk factor is the accumulation of fetal cells in the maternal thyroid (fetal microchimerism) with consequent development of an autoimmune response.27
Smoking is associated with about a 2- to 3-fold increased risk of Graves disease, and the risk of ophthalmopathy among smokers is four to five times that for nonsmokers.28,29 The risk increases with higher pack-years of smoking, and cessation results in a progressive decline over time. The mechanisms of increased risk are uncertain at this time, but considerations include tissue damage because of increased generation of reactive oxygen species, increased production of autoantibodies, and increased concentration of soluble adhesion molecules.30
Infectious microorganisms have been implicated as etiologic agents because of antigen mimicry or other mechanisms resulting in autoimmunity. Yersinia enterocolitica is high on the list of suspects for causing Graves disease. It possesses one or more outer membrane proteins that have homology with TSHR and studies have revealed significantly higher levels of antibodies to Y. enterocolitica among patients with Graves disease.17 However, most individuals with Y. enterocolitica infection do not develop thyroid disease, and a definitive link to it or other infectious agents has not been established at this time.
Graves disease has genetic risk factors, but no single gene has been identified as causative or necessary. The concordance rate for monozygotic twins is 20% to 35%, whereas the concordance rate for dizygotic twins is <5%.12 This not only supports a genetic role but also indicates the involvement of other factors. The human leukocyte antigen (HLA) gene HLA-DR3 has been identified as a major susceptibility gene and is discussed below. Individuals with HLA-DR3, typically of European origin, have a relative risk in the range of 3 to 4.18
Pathogenesis and Molecular Genetics
Hyperthyroidism of Graves disease is due to the production of autoantibodies to the TSHR on follicular cells. The autoantibodies activate the receptor and stimulate thyroid hormone synthesis and secretion in addition to diffuse proliferation of the follicular epithelium. Before recognition as autoantibodies, they were called LATS. This term derived from the observation that sera of patients with Graves disease stimulated the thyroids of test animals for a longer time than TSH.8 Further studies revealed that the long-acting stimulator was an immunoglobulin (thyroid-stimulating immunoglobulin) that binds to the extracellular domain of TSHR. TSHR is a member of the superfamily of G protein-coupled receptors. The gene coding for the protein was cloned in 1989 and maps to chromosome 14q31.31,32,33 TSHR is composed of three domains, one extracellular, one intracellular, and a connecting seven-loop transmembrane domain. The extracellular domain is relatively large, accounting for approximately half of the molecular size of the receptor, and serves as the binding site for TSH.34 The binding of TSH to TSHR results in stimulation of second messenger pathways predominately through cyclic AMP (cAMP) with eventual effect on the gene expression of follicular cells (see Chapter 8, Fig. 8.2).
Antibodies to TSHR attach to epitopes in the region of the TSH-binding pocket of the extracellular domain and can have stimulatory, blocking, or neutral effects on thyroid function.35,36,37 Stimulatory antibodies are specific for Graves disease, and these antibodies are detectable in almost all cases using modern assays. Blocking antibodies are more typically found in chronic lymphocytic (Hashimoto) thyroiditis and can contribute to hypothyroidism by preventing the binding of TSH. Both stimulatory and blocking antibodies may be detected in some cases of Graves disease. These stimulatory and blocking antibodies share comparable features of low serum concentration, high affinity, and similar binding epitopes on TSHR.38
A relatively unique aspect of TSHR is the phenomenon of molecular cleavage with shedding of the α subunit. This subunit constitutes the majority of the extracellular domain, and shedding exposes epitopes in the TSHR region to antibodies that may be partially hindered sterically when the receptor is attached to the follicular cell membrane.39 The shedding of the α subunit may play a role in the initiation or amplification of the autoimmune response. Follicular cells may also contribute to increased vascularity of the thyroid by secreting vascular endothelial growth factor in response to stimulation by TSHR autoantibodies.40
A complex interaction of T and B lymphocytes and thyroid follicular cells underlies the pathogenic autoantibody response.41 Our knowledge of the extent and specifics of the various immunologic mechanisms is incomplete, but it seems clear that T lymphocytes play an important role in the pathogenesis. Thyroids with Graves disease contain CD8+ and CD4+ T cells including CD4+ Th1, CD4+ Th2, and CD4+ Treg (CD25+) subsets. The main functions of CD4+ Th1 cells are related to cell-mediated immune responses, whereas CD4+ Th2 cells are involved predominately in antibody production. Graves disease is primarily a Th2 type of autoimmune disease because of the role of autoantibodies to TSHR. Stimulation of either the Th1 or the Th2 pathway results in reciprocal inhibition of the other. The CD4+ CD25+ Treg cells have an inhibitory effect on both Th1 and Th2 cells.
During pregnancy, the immune system is shifted toward a Th2 response with consequent decrease in Th1, a seemingly paradoxical finding given that women with Graves disease frequently experience a decrease in severity. This suggests that immunomodulation mechanisms are more complex than a simple shift in the Th1:Th2 ratio and that much remains to be clarified in regard to the autoimmune mechanisms associated with Graves disease. However, it does seem clear that perturbation of the balance of immune response and inhibition with the net loss of self-tolerance is a basic feature of autoimmunity, and these lymphocytes appear to have prime roles in Graves disease.
Thyroid follicular cells also appear to play an active role in the autoimmune process. They are capable of major histocompatibility complex (MHC) class II (HLA-DR) expression owing to various stimuli, and one of the prime inducers is interferon γ(IFN-γ).42 Expression of HLA-DR antigens could result from cytokines produced by infection (e.g., viral-induced IFN production) or other environmental factors with the subsequent initiation of an autoimmune response.
HLA genes of the MHC located on chromosome 6p21 are associated with Graves disease, particularly HLA-DR3 (HLA-DRB1*03).43 Increased risk of Graves disease is particularly associated with an arginine residue at position 74 of the DR β-1 chain.44 The threedimensional configuration of the peptide-binding pocket is different with the presence of a positively charged arginine residue compared with a neutral alanine or glutamine residue. A recent study of an ethnic Han Chinese population revealed Graves disease-associated HLA alleles different from those associated with Caucasian populations, with HLA-DPB1*05:01 identified as the major susceptibility gene.45
The risk of Graves disease also developing in an HLA-matched sibling is lower than that of a monozygotic twin, supporting the concept that genes other than HLA alleles are involved.46 CTLA-4 is a non-HLA gene whose product downregulates T-cell-mediated immune response and is important for maintaining selftolerance. Polymorphisms of this gene have been associated with autoimmune diseases including Graves disease.47 Other immune regulatory genes have been implicated in the pathogenesis of Graves disease including CD40 and protein tyrosine phosphatase-2 (PTPN2).43 The CD40 gene, located on chromosome 20q,
has also been linked to Graves disease. CD40 is a member of the tumor necrosis factor receptor superfamily and is expressed on B lymphocytes and antigen presenting cells. It functions as a costimulatory protein that is involved in various immune responses, and a polymorphism that results in increased CD40 expression could augment the development of autoimmunity.48 PTPN2 codes for a lymphoid tyrosine phosphatase that is an inhibitor of T-cell activation. A single-nucleotide polymorphism at codon 620 in which tryptophan is substituted for arginine has been found to be linked to Graves disease and other autoimmune disorders.48,49 Several other genes have shown association with Graves disease in some populations, and it is highly likely that others will be identified with the broader application of newer molecular pathology techniques.
has also been linked to Graves disease. CD40 is a member of the tumor necrosis factor receptor superfamily and is expressed on B lymphocytes and antigen presenting cells. It functions as a costimulatory protein that is involved in various immune responses, and a polymorphism that results in increased CD40 expression could augment the development of autoimmunity.48 PTPN2 codes for a lymphoid tyrosine phosphatase that is an inhibitor of T-cell activation. A single-nucleotide polymorphism at codon 620 in which tryptophan is substituted for arginine has been found to be linked to Graves disease and other autoimmune disorders.48,49 Several other genes have shown association with Graves disease in some populations, and it is highly likely that others will be identified with the broader application of newer molecular pathology techniques.
Ophthalmopathy
Ophthalmopathy is the most frequent and significant extrathyroidal manifestation of Graves disease.50 The extraocular muscles become inflamed and edematous, and the orbital fibroadipose tissue and lacrimal glands increase in volume. The extraocular muscles and fibroadipose tissue are infiltrated by lymphocytes, predominately T cells, and the tissues accumulate excessive amounts of hydrophilic glycosaminoglycans that derive from fibroblasts. Over time, the extraocular muscles can become atrophic and surrounded by fibrotic tissue.
The basic pathogenic mechanism appears to be autoantibodies directed at orbital tissues.51 Orbital preadipocyte fibroblasts express TSHR, and stimulation by autoantibodies results in the generation of increased adipose tissue. Autoantibodies to insulinlike growth factor receptor have also been implicated. Stimulation of this receptor causes glycosaminoglycans production along with T-cell recruitment and activation.
Clinical Presentation and Imaging
Presenting signs and symptoms of Graves disease parallel those of increased sympathetic nervous system activity. Common symptoms include nervousness, anxiety, fatigue, heat hypersensitivity, increased perspiration, palpitations, and increased appetite but with loss of weight. Physical examination typically reveals warm moist skin, tremor, tachycardia, hypertension, muscle weakness, lid lag and stare, and mild to moderate goiter. A personal or family history of autoimmune disease is common. Scintigraphic imaging studies with radioiodine reveal diffusely elevated uptake (Fig. 5.1).
Laboratory Tests
Thyroid function tests reveal elevated free T4 in most cases of Graves disease. However, a normal free T4 concentration can be found in a small percentage of cases, and thus, measurement of free T3 may be necessary to demonstrate elevated hormone production. TSH is abnormally low or undetectable and is particularly useful in the evaluation of hyperthyroidism.
Antibodies to TSH receptors are detectable in almost all untreated cases and are highly specific with few false positive results using modern techniques. Two assays are used to detect TSHR antibodies, one measuring TSH-binding inhibitory immunoglobulins (TBII) and the other thyroid-stimulating antibodies. The two assays do not yield totally comparable results because TSHR autoantibodies are heterogeneous and can have agonistic, antagonistic, or neutral effects on the receptor.35 Both stimulating and blocking antibodies are detected by the TBII assay, and blocking antibodies can interfere with the detection of stimulating antibodies.
The TBII test is an immunoassay that measures inhibition of TSH binding to TSH receptors. Current methodology uses solubilized TSH receptors and an enzymatic detection system. Alternative names for the TBII test include thyroid receptor antibody and LATS.
Stimulating antibodies are detected by a bioassay measuring the release of cAMP from thyroid membranes or cell lines transfected with the TSH receptor. This test is often known by the acronym TSI (thyroid-stimulating immunoglobulin) or TSAb (thyroid-stimulating antibody). This same basic bioassay can be utilized to detect thyroid-blocking antibodies by adding TSH to both patient and control sera and then comparing the relative release of cAMP. The presence of blocking antibodies results in reduced detection of cAMP.
After therapy with radioiodine, the frequency of TSHR antibody detection decreases over the following decade, although there may be an initial transient increase.52 Antibodies to thyroperoxidase (TPO) are found in most cases, and about half have antibodies to thyroglobulin (TG). However, antibodies to TPO and TG are relatively nonspecific and of limited utility in the evaluation of Graves disease.
Pathology
Gross Features
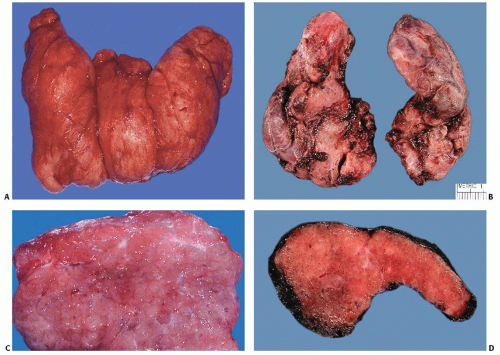
Thyroid glands show mild to moderate diffuse symmetrical enlargement with a weight usually in the 50 to 150 g range (Fig. 5.2). The color of the cut surface can range from red to reddish brown or tan to a pinkish gray appearance, the variation in color reflective of differing degrees of vascularity. Untreated cases have high vascularity and a dark red appearance, whereas treated cases appear lighter and more similar to normal thyroid because of decreased vascularity and the accumulation of colloid. The consistency ranges from spongy to firm. Various culinary adjectives such as succulent, meaty, or beefy have been used in descriptions, and untreated thyroids bear resemblance to skeletal muscle. Vague nodularity may be seen, but fibrous septa are absent or inconspicuous.
Histologic Features
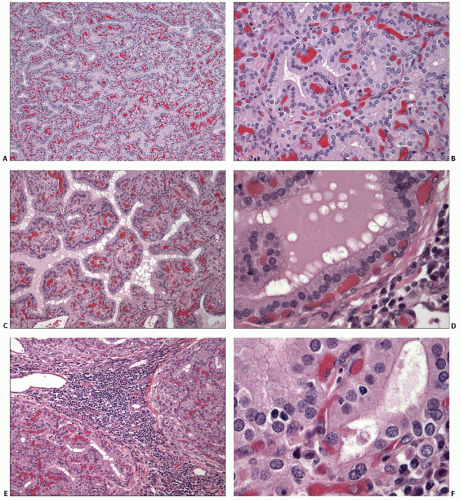
Diffuse hyperplasia of follicular epithelium is the characteristic histologic finding. The follicles are lined by tall columnar cells that exhibit papillary infolding within the lumina. The papillae are generally simple and nonbranching with limited projection into the central space. In cases with florid hyperplasia, such as those with little or no treatment, papillae may be more prominent and occupy a large portion of the lumen (Fig. 5.3).
Untreated cases contain very little colloid; however, some form of therapy precedes the resection of most cases, and colloid is usually seen. The amount of colloid can vary in different areas depending on the degree of epithelial hyperplasia (Fig. 5.4). Although some variability in the degree of hyperplasia and amount of colloid is typically seen from area to area, the hyperplastic change is diffuse and identifiable in all sections obtained from one or both lobes.
Colloid usually stains lighter and may appear quite pale compared with that in normal thyroids. Scalloping is seen at the interface of the colloid with the follicular cells, particularly in areas with hyperplastic papillae. Cytoplasm of follicular cells can be eosinophilic, amphophilic, microvacuolated, or even clear because of lipid or glycogen accumulation (Fig. 5.5).
Follicular cells contain round to oval, basally located nuclei with finely granular chromatin. Small nucleoli can be seen in some cells. Most nuclei are normochromatic and mildly increased in size. Only mild variation in nuclear size and shape is typically seen. The prime exception is nuclear atypia after radioiodine therapy (Fig. 5.6). Some nuclei may appear hyperchromatic or hypochromatic, and the latter can cause concern for papillary carcinoma. However, the degree of hypochromasia is usually less than that seen in papillary carcinoma, and the nuclei lack other features characteristic of malignancy.
Lymphocytes are commonly seen in the interfollicular stroma. The distribution and total quantity is variable in a given thyroid, ranging from scant or absent to focally dense with germinal centers. Fibrous tissue is usually absent or scant with the exception of some long-standing cases or those treated with radioactive iodine.
Immunohistochemistry
Immunostains have limited diagnostic applicability in Graves disease. Cells typically reveal proliferative activity below 5% as measured by Ki-67 labeling and retain p27 immunoreactivity in most nuclei.53 Focal lesions questionable for neoplasia can be analyzed with appropriate stains (see Chapter 11). Most lymphocytes infiltrating the thyroid are T lymphocytes with both CD4+ and CD8+ cells demonstrable. Overall, CD8+ lymphocytes tend to outnumber CD4+ lymphocytes, and the cells infiltrating the follicular epithelium are predominately CD8+.54,55,56
CD4+ lymphocytes predominate in the interfollicular stroma.57 CD20+ B cells comprise the majority of lymphocytes in germinal centers. The perifollicular regions contain a mixture of CD4+ and CD8+ T lymphocytes with the latter more frequent. Expression of HLA-DR can be demonstrated in both lymphocytes and follicular cells.58
CD4+ lymphocytes predominate in the interfollicular stroma.57 CD20+ B cells comprise the majority of lymphocytes in germinal centers. The perifollicular regions contain a mixture of CD4+ and CD8+ T lymphocytes with the latter more frequent. Expression of HLA-DR can be demonstrated in both lymphocytes and follicular cells.58
Effects of Therapy
Most surgically resected thyroid glands have been subject to prior treatment with antithyroid drugs or radioactive iodine. Rarely does a surgical pathologist examine an untreated gland. Methimazole and propylthiouracil are common antithyroid drugs that act primarily by blocking hormone synthesis through
interference with peroxidase-mediated iodination of tyrosyl residues. Thyroid glands may show regression of hyperplastic changes, but the regression is typically incomplete with focal areas of follicular hyperplasia still present. β-Adrenergic blockers such as propranolol do not induce appreciable morphologic changes in the thyroid.59 Nonradioactive iodine therapy was common in the past but unusual today as a first-line or sole medication. Iodine inhibits release of thyroid hormones and peripheral conversion of T4 to T3, the biologically active form of thyroid hormone. Nonradioactive iodine reduces thyroid vascularity, increases colloid stores, and promotes involution of the follicular epithelium. Owing to its reduction of vascularity,
nonradioactive iodine is commonly administered before surgical resection.
interference with peroxidase-mediated iodination of tyrosyl residues. Thyroid glands may show regression of hyperplastic changes, but the regression is typically incomplete with focal areas of follicular hyperplasia still present. β-Adrenergic blockers such as propranolol do not induce appreciable morphologic changes in the thyroid.59 Nonradioactive iodine therapy was common in the past but unusual today as a first-line or sole medication. Iodine inhibits release of thyroid hormones and peripheral conversion of T4 to T3, the biologically active form of thyroid hormone. Nonradioactive iodine reduces thyroid vascularity, increases colloid stores, and promotes involution of the follicular epithelium. Owing to its reduction of vascularity,
nonradioactive iodine is commonly administered before surgical resection.
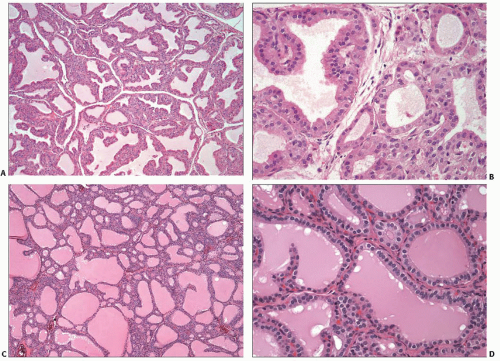 FIGURE 5.4. Graves disease, treated. Two cases treated with antithyroid drugs before resection (A and B) and (C and D). Both cases contain more colloid and less papillary hyperplasia compared to the untreated case shown in Fig. 5.3. |
Radioactive iodine therapy is associated with various histopathologic features. The range of findings includes follicular atrophy, fibrosis, oncocytic metaplasia, nuclear atypia, formation of hyperplastic nodules, and persistence of lymphocytic infiltrates.60,61 A diagnosis of nodular hyperplasia or chronic lymphocytic (Hashimoto) thyroiditis may be considered by a pathologist unaware of the clinical diagnosis and prior treatment.
Nodules
Nodular lesions are found in 10% to 25% of thyroids with Graves disease, and most are benign with features of follicular hyperplasia or colloid nodule. However, about 10% to 20% of nodules are found to have carcinoma.62,63,64,65 The overwhelming majority of carcinomas are papillary thyroid carcinoma, and many are incidental microcarcinomas. The overall incidence of carcinoma in Graves disease is 1% to 9% with large, more recent studies yielding results in the range of 1% to 4%.62,63,64,65 Lymphoma rarely occurs in association with Graves disease.
Cytologic Features
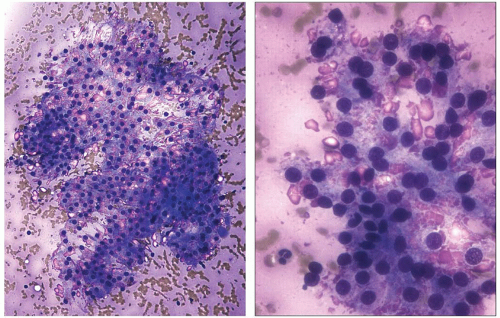
Fine needle aspiration (FNA) usually yields scant material that appears bloody. Microscopic examination of smears typically shows low to moderate cellularity with collections of follicular cells admixed with blood. Colloid is scant or difficult to recognize because of its thin, pale appearance, particularly in Papanicolaou-stained slides. Follicular cells are usually arranged in flat sheets or microfollicles, and papillae are typically few or absent.
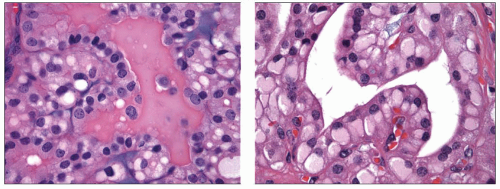
The cytoplasm of follicular cells is increased and usually has a pale finely granular appearance. Clusters of cells may exhibit marginal vacuolization of their cytoplasm. These vacuoles are clear in Papanicolaou-stained slides but have a pink granular appearance in Diff-Quik-stained preparations (Fig. 5.7). These vacuolated cells are known as flame, fire, or flare cells. Although initially described as distinctive for Graves disease, these cells can be seen in cases of nodular hyperplasia, follicular neoplasia, and papillary carcinoma.66
Nuclei are generally round and mildly enlarged. Variation in size may be seen with some nuclei having a diameter several times that of a normal follicular cell. Small nucleoli are common. Lymphocytes and/or oncocytic cells are seen in about 40% to 50% of aspirates.67 Less frequently, multinucleated giant cells and clusters of epithelioid macrophages may be present. Aspirates of glands after treatment with antithyroid drugs or radioiodine may show cytologic atypia suggestive of malignancy, particularly papillary thyroid carcinoma.68,69
Cases of Graves disease are seldom aspirated because the diagnosis is usually based on clinical findings and confirmatory laboratory tests. Aspiration is typically performed when a nodular lesion is detected, and thus, the cytologic findings are likely to be those of another thyroid lesion such as nodular hyperplasia, papillary carcinoma, or follicular neoplasia. Features of Graves disease may be absent or only evident in a minority of the cell population.
Differential Diagnosis
The clinical differential diagnosis of thyrotoxicosis comprises various conditions including those caused by hyperactivity of the
thyroid gland (hyperthyroidism) and those caused by extrathyroidal abnormalities (Table 5.1). Morphologically, the differential diagnosis includes thyroid neoplasia, particularly papillary carcinoma, chronic lymphocytic thyroiditis with hyperthyroidism (hashitoxicosis), and toxic nodular hyperplasia. Acquisition of clinical history and laboratory test results is an important step in the evaluation of these thyroids. The effects of treatment may transform a diffusely hyperplastic thyroid to one with features similar to other conditions.
thyroid gland (hyperthyroidism) and those caused by extrathyroidal abnormalities (Table 5.1). Morphologically, the differential diagnosis includes thyroid neoplasia, particularly papillary carcinoma, chronic lymphocytic thyroiditis with hyperthyroidism (hashitoxicosis), and toxic nodular hyperplasia. Acquisition of clinical history and laboratory test results is an important step in the evaluation of these thyroids. The effects of treatment may transform a diffusely hyperplastic thyroid to one with features similar to other conditions.
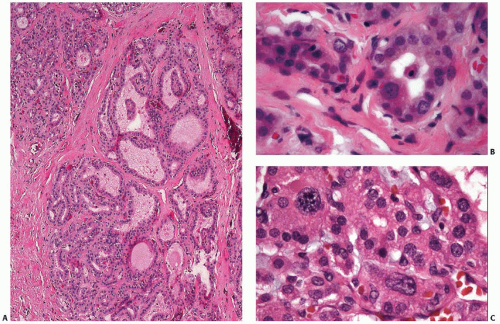
On a purely histologic basis, differentiation from papillary carcinoma poses the greatest challenge because of the presence of papillae and, in some cases, hypochromatic nuclei (Fig. 5.8 and Table 5.2). Even psammoma bodies have been reported in Graves disease, although this seems to be a rare observation.70 The absence of both well-developed fibrovascular cores in hyperplastic papillae and the broader range of nuclear features characteristic of papillary thyroid carcinoma combined with the diffuse nature of hyperplastic changes are the key features for excluding a neoplastic process. Immunostaining for p27 may be of help because it shows positivity in most nuclei in Graves disease, whereas it is frequently lost in nuclei of papillary carcinoma cells.53 Distinct nodular lesions require more scrutiny, and immunostains such as galectin-3 and HBME-1 may be helpful (see Chapter 11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree