Chapter 42 Cell differentiation and ergastic cell contents
Modifications to the basic structure of the living plant cell involving composition of the cell wall, cell shape and cell contents, are found in the various plant tissues and furnish those microscopical characters of drug plants which are of value in identification and in the detection of adulteration.
THE CELL WALL
Cellulose walls
Lignified walls
Lignin is a strengthening material which impregnates the cell walls of tracheids, vessels, fibres and sclereids of vascular plants; it constitutes 22–34% of woods. Chemically, it is a complex phenylpropanoid (C6–C3) polymer which differs according to its source, lignin from dicotyledons being different from that of the conifers (Fig. 21.1). In the wall, it appears to occur chemically combined with hemicellulose and is built up in greatest concentration in the middle lamellae and in the primary walls. Lignified cell walls after treatment with Schultze’s macerating fluid will show cellulose reactions.
For the identification of lignified walls the following tests are available:
Suberized and cutinized walls
Suberin and cutin consist of mixtures of substances, chiefly highly polymerized fatty acids such as suberic acid, COOH[CH2]6COOH, although the acids present in the two substances are not identical. These materials waterproof cells in which they occur. Suberin thickenings, such as are found in cork cells and endodermal cells, usually consist of carbohydrate-free suberin lamellae. Cutin forms a secondary deposit on or in a cellulose wall. Leaves are frequently covered with a deposit of cutin which may show characteristic papillae, ridges or striations. Beneath the cuticle, the cellulose wall may also be impregnated with cutin (cutinized), so that these walls may show a gradation from pure cellulose on the inside, through layers of cellulose impregnated with pectin compounds and fatty substances, to the outer cuticle, which is free of cellulose. Waxes (largely esters of higher monohydric alcohols and fatty acids) occur with suberin and cutin. Unlike the latter, they readily melt on warming and are extractable with fat solvents. Such waxes in the form of minute rods or particles give a glaucous effect to the structures which they cover and are responsible for the ‘bloom’ of many fruits, stems, etc. Wax is found in larger amounts on the leaves of Myrica, and in the wax palms, Copernicia, it coats the leaves heavily (Carnauba wax).
The reactions of suberin and cutin are almost identical.
THE EPIDERMIS
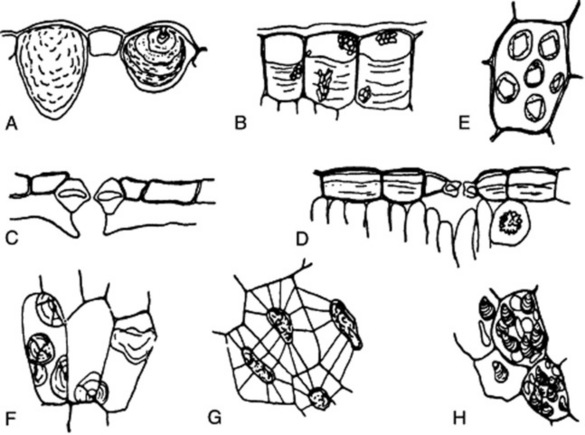
For leaves in particular, the shape of the epidermal cells in surface view and in section (Fig. 42.1A–D), the nature and distribution of the wall thickening, the presence or absence of cuticle and its form, the distribution and structure of the stomata, the presence or absence of well-differentiated subsidiary cells to the stomata, the presence of characteristic cell inclusions such as cystoliths, the presence or absence and form, size and distribution of epidermal trichomes and the presence and distribution of water-pores should all be carefully noted in describing the characters of an epidermis.
The structures of the epidermis and stomata are of first importance in the microscopical identification of leaves (see Fig. 42.2). Straight-walled epidermal cells are seen in, for example, jaborandi, coca and senna leaves; wavy-walled epidermal cells in stramonium, hyoscyamus and belladonna; beaded walls in Lobelia inflata and Digitalis lanata; a papillose epidermis in coca leaf. A thick cuticle is developed in Aloe leaf and bearberry leaf; a striated cuticle in belladonna, jaborandi, Digitalis lutea and D. thapsi. Mucilage is present in the epidermis of senna and buchu leaves. Cystoliths of calcium carbonate occur in the epidermal cells of Urticaceae and Cannabinaceae; sphaero-crystals of diosmin occur in buchu epidermis (Fig. 42.1B).
The stomata may be surrounded by cells resembling the other epidermal cells (anomocytic, formerly ranunculaceous, type), but in other cases definite subsidiary cells may be distinguished. Three main types are distinguishable: the anisocytic (formerly cruciferous) type, with the stoma surrounded by three or four subsidiary cells, one of which is markedly smaller than the others; the paracytic (formerly rubiaceous) type, with two subsidiary cells with their long axes parallel to the pore; and the diacytic (formerly caryophyllaceous) type with two subsidiary cells, with their long axis at right angles to the pore of the stomata (Fig. 42.2). There are variations among these types (e.g. the actinocytic type, in which the subsidiary cells are arranged along the radii of a circle) and altogether some 31 types have been recognized. (For a survey of the classification of morphological types of stomata see M. Baranova, Bot. Rev., 1992, 58, 49).
Often, when viewed under the light microscope as cleared preparations, the outlines of the epidermal cells and stomata do not appear as definite as the line drawings (Fig. 42.2) might suggest. This is due tothe convoluted arrangements of cells on the leaf surface and is illustrated by the scanning electron micrographs included in the digitalis and Solanaceae descriptions in Part 5.
The distribution of stomata between the upper and lower epidermis shows great variation. The stomata may be entirely confined to the lower epidermis, as in Ficus species, bearberry, boldo, buchu, coca, jaborandi and maté leaves. The leaves of savin show stomata confined to two localized areas of the lower surface. The floating leaves of aquatics have stomata confined to the upper epidermis. Sometimes they are evenly distributed on both surfaces; most commonly they are more numerous on the lower surface. For ‘stomatal number’ and ‘stomatal index’ see Chapter 43.
The epidermis of fruits and seeds may yield characters of diagnostic value (see Fig. 41.7). The outer and inner epidermi of the pericarp of the umbelliferous fruits are highly characteristic structures. Characteristic cells with thickened pitted walls form the outer epidermis of the pericarp in vanilla, juniper and capsicum. The outer epidermi of the pericarp of coriander and vanilla contain prisms of calcium oxalate. A striated cuticle is seen in aniseed, caraway and star anise fruits. Thickened palisade-like cells form the epidermis of the testa of colocynth and fenugreek seeds. Characteristic elongated tapering cells form the epidermis of cardamoms. Thickened lignified cells form the epidermis of lobelia seed, and mucilage cells that of linseed and of white and black mustard.
EPIDERMAL TRICHOMES
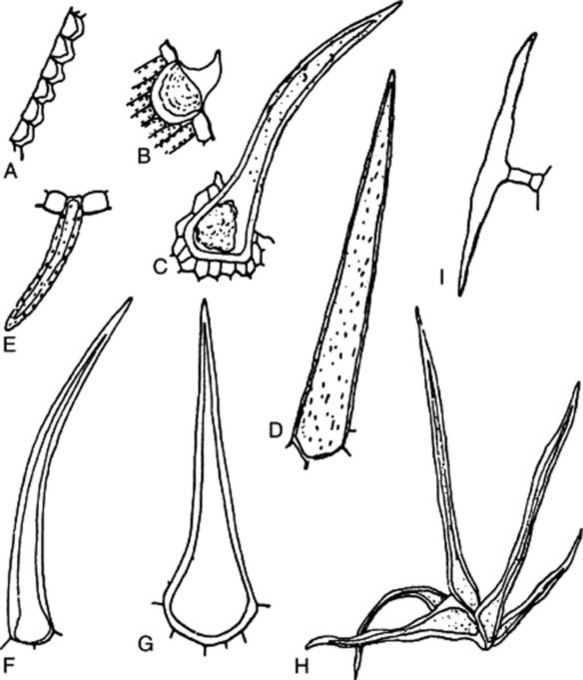
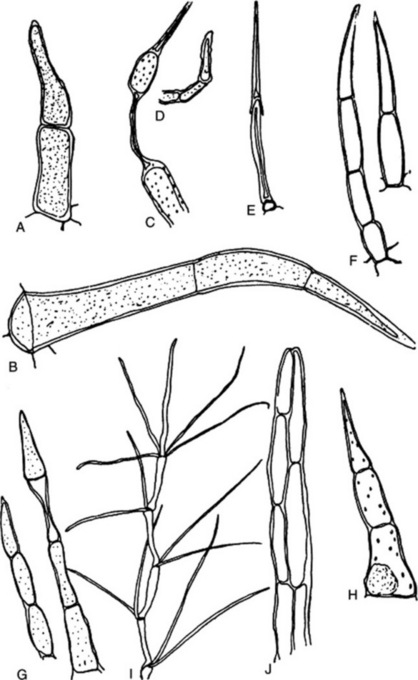
Most leaves and many herbaceous stems, flowers, fruits and seeds possess hairs or trichomes of one kind or another. Many show hairs of more than one type. Hairs may be grouped into non-glandular or clothing hairs, and glandular hairs. Clothing hairs may be unicellular or multicellular. Unicellular hairs vary from small papillose outgrowths to large robust structures (Figs 42.3, 42.4). Multicellular hairs may be uniseriate, biseriate or multiseriate or complicated branched structures (Fig. 42.4). The chemical nature of the cell wall, and the presence of pits or protuberances or of cell inclusions, such as cystoliths, should be noted.
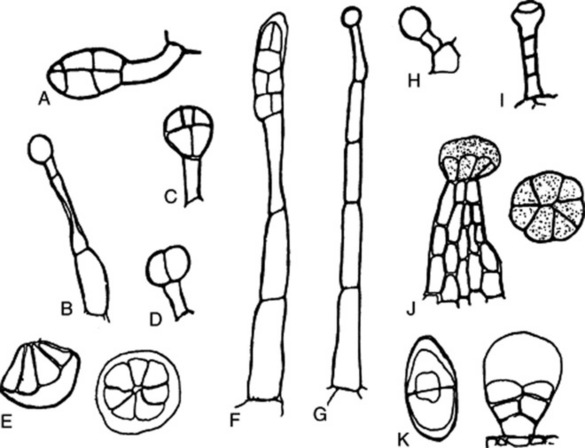
Glandular hairs may have a unicellular or a multiseriate stalk; the glandular head may be unicellular or multicellular (Fig. 42.5). The cuticle of the gland may be raised by the secretion (Fig. 42.5E and F). In peppermint the oil secretion beneath the cuticle contains crystals of menthol. A particular type of hair is often characteristic of a plant family or genus—for example, biseriate hairs of the form shown in Fig. 42.4J are common in the Compositae, while glandular hairs such as Fig. 42.5A, B and C are found in the Solanaceae, and such as Fig. 42.5E in the Labiatae. For types of hairs found on seeds, see sections on cotton, strophanthus seeds and nux vomica seeds.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree