CHAPTER 21 Diencephalon
The diencephalon is part of the prosencephalon (forebrain), which develops from the foremost primary cerebral vesicle and differentiates into a caudal diencephalon and rostral telencephalon. The cerebral hemispheres develop from the sides of the telencephalon, each containing a lateral ventricle. The sites of evagination become the interventricular foramina, through which the two lateral ventricles and midline third ventricle communicate. The diencephalon corresponds largely to the structures that develop lateral to the third ventricle. The lateral walls of the diencephalon form the epithalamus most superiorly, the thalamus centrally and the subthalamus and hypothalamus most inferiorly.
THALAMUS
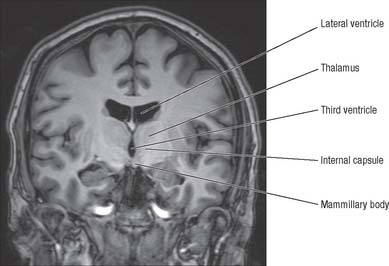
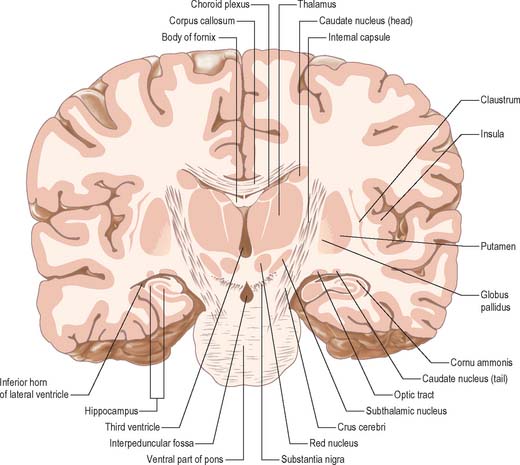
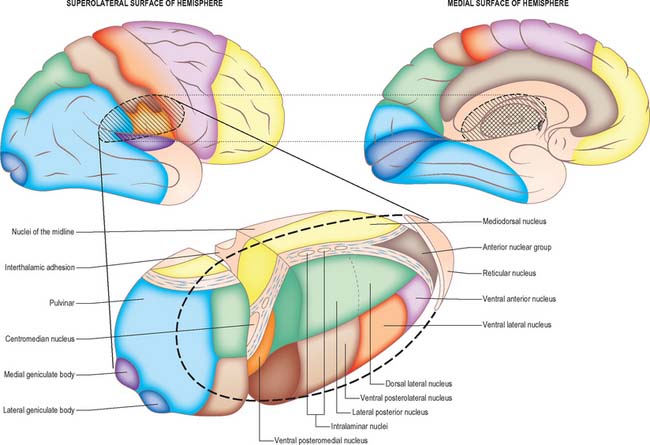
The thalamus is an ovoid nuclear mass, about 4 cm long, which borders the dorsal part of the third ventricle (Figs 21.1, 21.2). The narrow anterior pole lies close to the midline, and forms the posterior boundary of the interventricular foramen. Posteriorly, an expansion, the pulvinar, extends beyond the third ventricle to overhang the superior colliculus (see Fig. 19.3). The brachium of the superior colliculus (superior quadrigeminal brachium) separates the pulvinar above from the medial geniculate body below. A small oval elevation, the lateral geniculate body, lies lateral to the medial geniculate.
The superior (dorsal) surface of the thalamus (see Fig. 19.3) is covered by a thin layer of white matter, the stratum zonale. It extends laterally from the line of reflection of the ependyma (taenia thalami), and forms the roof of the third ventricle. This curved surface is separated from the overlying body of the fornix by the choroid fissure with the tela choroidea within it. More laterally, it forms part of the floor of the lateral ventricle. The lateral border of the superior surface of the thalamus is marked by the stria terminalis and overlying thalamostriate vein, which separate the thalamus from the body of the caudate nucleus. Laterally, a slender sheet of white matter, the external medullary lamina, separates the main body of the thalamus from the reticular nucleus. Lateral to this, the thick posterior limb of the internal capsule lies between the thalamus and the lentiform complex.
The medial surface of the thalamus is the superior (dorsal) part of the lateral wall of the third ventricle. It is usually connected to the contralateral thalamus by an interthalamic adhesion behind the interventricular foramina (see Fig. 16.7). The boundary with the hypothalamus is marked by an indistinct hypothalamic sulcus, which curves from the upper end of the cerebral aqueduct to the interventricular foramen. The thalamus is continuous with the midbrain tegmentum, the subthalamus and the hypothalamus.
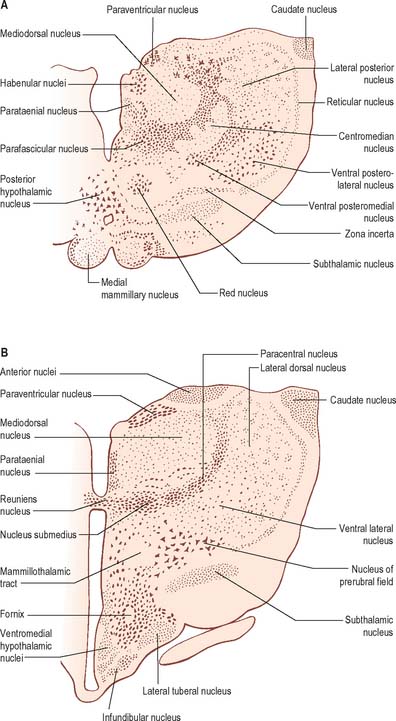
Internally, the greater part of the thalamus is divided into anterior, medial and lateral nuclear groups by a vertical Y-shaped sheet of white matter, the internal medullary lamina (Figs 21.3, 21.4). In addition, intralaminar nuclei lie embedded within the internal medullary lamina and midline nuclei abut the lateral walls of the third ventricle. The reticular nucleus forms a shell-like lateral covering to the main nuclear mass, separated from it by an external medullary lamina of nerve fibres.
In general, thalamic nuclei both project to and receive fibres from the cerebral cortex (Fig. 21.4). The whole cerebral cortex, including neocortex, paleocortex of the piriform lobe and archicortex of the hippocampal formation, is reciprocally connected with the thalamus. The thalamus is the major route by which subcortical neuronal activity influences the cerebral cortex, and the greatest input to most thalamic nuclei comes from the cerebral cortex.
ANTERIOR THALAMIC NUCLEI
The anterior group of nuclei are enclosed between the arms of the Y-shaped internal medullary lamina, and underlie the anterior thalamic tubercle (see Figs 19.3, 21.4). Three subdivisions are recognized: the largest is the anteroventral nucleus, the others being the anteromedial and anterodorsal nuclei.
MEDIAL THALAMIC NUCLEI
The mediodorsal nucleus appears to be involved in a wide variety of higher functions. Damage may lead to a decrease in anxiety, tension, aggression or obsessive thinking. There may also be transient amnesia, with confusion developing particularly over the passage of time. Much of the neuropsychology of medial nuclear damage reflects defects in functions similar to those performed by the prefrontal cortex, with which it is closely linked. The effects of ablation of the mediodorsal nuclei parallel, in part, the results of prefrontal lobotomy.
LATERAL THALAMIC NUCLEI
Ventral lateral nucleus
Approximately 70% of VL neurones are large relay neurones, and the other 30% are local circuit interneurones. Responses can be recorded in VL thalamus during both passive and active movement of the contralateral body. The topography of its connections, and recordings made within the nucleus, suggest that VLc contains a body representation comparable to that in the ventral posterior nucleus. In patients with tremor, clusters of VL neurones fire in bursts that are synchronous with peripheral tremor: these cells have been designated as ‘tremor cells’. Stereotaxic surgery of the ventral lateral nucleus is sometimes used in the treatment of essential tremor. In the past, thalamotomy was used extensively for the treatment of Parkinson’s disease. The internal segment of the globus pallidus and the subthalamic nucleus are now the preferred neurosurgical targets for Parkinson’s disease (see Ch. 22).
Medial geniculate nucleus
The medial geniculate nucleus, which is a part of the auditory pathway (see Ch. 37), is located within the medial geniculate body, a rounded elevation situated posteriorly on the ventrolateral surface of the thalamus, and separated from the pulvinar by the superior quadrigeminal brachium. It receives fibres travelling in the inferior quadrigeminal brachium. Three major subnuclei, medial, ventral and dorsal, are recognized within it. The inferior brachium separates the medial (magnocellular) nucleus, which consists of sparse, deeply staining neurones, from the lateral nucleus, which is made up of medium-sized, densely packed and darkly staining cells. The dorsal nucleus overlies the ventral nucleus and expands posteriorly; it is, therefore, sometimes known as the posterior nucleus of the medial geniculate. It contains small- to medium-sized, pale-staining cells, which are less densely packed than those of the lateral nucleus. The ventral nucleus receives fibres from the central nucleus of the ipsilateral inferior colliculus via the inferior quadrigeminal brachium and also from the contralateral inferior colliculus. The nucleus contains a complete tonotopic representation. Low-pitched sounds are represented laterally, and progressively higher-pitched sounds are encountered as the nucleus is traversed from lateral to medial. The dorsal nucleus receives afferents from the pericentral nucleus of the inferior colliculus and from other brain stem nuclei of the auditory pathway. A tonotopic representation has not been described in this subdivision and cells within the dorsal nucleus respond to a broad range of frequencies. The magnocellular medial nucleus receives fibres from the inferior colliculus and from the deep layers of the superior colliculus. Neurones within the magnocellular subdivision may respond to modalities other than sound. However, many cells respond to auditory stimuli, usually to a wider range of frequencies than neurones in the ventral nucleus. Many units show evidence of binaural interaction, with the leading effect arising from stimuli in the contralateral cochlea. The ventral nucleus projects primarily to the primary auditory cortex. The dorsal nucleus projects to auditory areas surrounding the primary auditory cortex. The magnocellular division projects diffusely to auditory areas of the cortex and to adjacent insular and opercular fields.
Lateral geniculate nucleus
The lateral geniculate body, which is part of the visual pathway (see Ch. 40), is a small, ovoid, ventral projection from the posterior thalamus (Fig. 21.5). The superior quadrigeminal brachium enters the posteromedial part of the lateral geniculate body dorsally, lying between the medial geniculate body and the pulvinar.
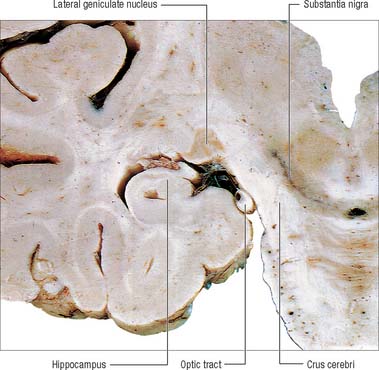
Fig. 21.5 Coronal section through the brain showing the lateral geniculate nucleus.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
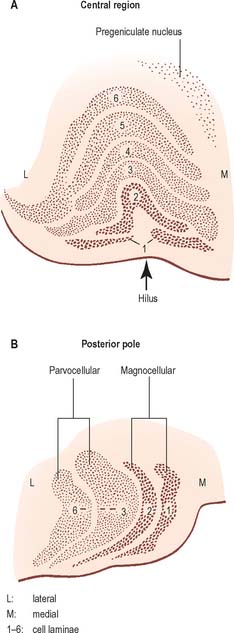
The lateral geniculate nucleus is an inverted, somewhat flattened, U-shaped nucleus and is laminated. Its internal organization is usually described on the basis of six laminae, although seven or eight may be present. The laminae are numbered 1 to 6, from the innermost ventral to the outermost dorsal (Fig. 21.6). Laminae 1 and 2 consist of large cells, the magnocellular layers, whereas layers 4 to 6 have smaller neurones, the parvocellular laminae. The apparent gaps between laminae are called the interlaminar zones. Most ventrally an additional superficial, or S, lamina is recognized.
The lateral geniculate nucleus receives a major afferent input from the retina. The contralateral nasal hemiretina projects to laminae 1, 4 and 6, whereas the ipsilateral temporal hemiretina projects to laminae 2, 3 and 5. The parvocellular laminae receive axons predominantly of X-type retinal ganglion cells, i.e. more slowly conducting cells with sustained responses to visual stimuli. The faster conducting, rapidly adapting Y-type retinal ganglion cells project mainly to the magnocellular laminae 1 and 2, and give off axonal branches to the superior colliculus. A third type of retinal ganglion cell, the W cells, which have large receptive fields and slow responses, project to both the superior colliculus and the lateral geniculate nucleus, where they terminate particularly in the interlaminar zones and in the S lamina.
The lateral geniculate nucleus is organized in a visuotopic manner and it contains a precise map of the contralateral visual field. The vertical meridian is represented posteriorly, the peripheral anteriorly, the upper field laterally, and the lower field medially. Similar precise, point-to-point, representation is also found in the projection of the lateral geniculate nucleus to the visual cortex. Radially arranged inverted pyramids of neurones in all laminae respond to a single, small area of the contralateral visual field and project to a circumscribed area of cortex. The termination of geniculocortical axons in the visual cortex is considered in detail elsewhere (see Ch. 23).
Pulvinar
The pulvinar corresponds to the posterior expansion of the thalamus, which overhangs the superior colliculus (Fig. 21.4). It has three major subdivisions, which are the medial, lateral and inferior pulvinar nuclei. The medial pulvinar nucleus is dorsomedial and consists of compact, evenly spaced, neurones. The inferior pulvinar nucleus lies laterally and inferiorly, and is traversed by bundles of axons in the mediolateral plane, an arrangement which confers a fragmented appearance of horizontal cords or sheets of cells separated by fibre bundles. The inferior pulvinar nucleus lies most inferiorly and laterally and is a more homogeneous collection of cells.
INTRALAMINAR NUCLEI
The term intralaminar nuclei refers to collections of neurones within the internal medullary lamina of the thalamus (Fig. 21.4). Two groups of nuclei are recognized. The anterior (rostral) group is subdivided into central medial, paracentral and central lateral nuclei. The posterior (caudal) intralaminar group consists of the centromedian and parafascicular nuclei. The designations central medial and centromedian are open to confusion; however, they are an accepted part of the terminology of thalamic nuclei in common usage. The centromedian nucleus is much larger, is considerably expanded in man in comparison with other species, and is importantly related to the globus pallidus, deep cerebellar nuclei and motor cortex. Anteriorly, the internal medullary lamina separates the mediodorsal nucleus from the ventral lateral complex. It is occupied by the paracentral nucleus laterally, and the central medial nucleus ventromedially, as the two laminae converge towards the midline. A little more posteriorly, the central lateral nucleus appears dorsally in the lamina as the latter splits to enclose the lateral dorsal nucleus. More posteriorly, at the level of the ventral posterior nucleus, the lamina splits to enclose the ovoid centromedian nucleus. The smaller parafascicular nucleus lies more medially.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree