Diarrhea, Constipation, and Irritable Bowel Syndrome
KEY CONCEPTS
![]() Diarrhea is caused by many viral and bacterial organisms. It is most often a minor discomfort, not life-threatening, and usually self-limited.
Diarrhea is caused by many viral and bacterial organisms. It is most often a minor discomfort, not life-threatening, and usually self-limited.
![]() The four pathophysiologic mechanisms of diarrhea have been linked to the four broad diarrheal groups, which are secretory, osmotic, exudative, and altered intestinal transit. The three mechanisms by which absorption occurs from the intestines are active transport, diffusion, and solvent drag.
The four pathophysiologic mechanisms of diarrhea have been linked to the four broad diarrheal groups, which are secretory, osmotic, exudative, and altered intestinal transit. The three mechanisms by which absorption occurs from the intestines are active transport, diffusion, and solvent drag.
![]() Management of diarrhea focuses on preventing excessive water and electrolyte losses, dietary care, relieving symptoms, treating curable causes, and treating secondary disorders.
Management of diarrhea focuses on preventing excessive water and electrolyte losses, dietary care, relieving symptoms, treating curable causes, and treating secondary disorders.
![]() Bismuth subsalicylate is marketed for indigestion, relieving abdominal cramps, and controlling diarrhea, including traveler’s diarrhea, but may cause interactions with several components if given excessively.
Bismuth subsalicylate is marketed for indigestion, relieving abdominal cramps, and controlling diarrhea, including traveler’s diarrhea, but may cause interactions with several components if given excessively.
![]() Constipation is defined as difficult or infrequent passage of stool, at times associated with straining or a feeling of incomplete defecation.
Constipation is defined as difficult or infrequent passage of stool, at times associated with straining or a feeling of incomplete defecation.
![]() Underlying causes of constipation should be identified when possible and corrective measures taken (e.g., alteration of diet or treatment of diseases such as hypothyroidism).
Underlying causes of constipation should be identified when possible and corrective measures taken (e.g., alteration of diet or treatment of diseases such as hypothyroidism).
![]() The foundation of treatment of constipation is dietary fiber or bulk-forming laxatives that provide 20 to 25 g/day of raw fiber.
The foundation of treatment of constipation is dietary fiber or bulk-forming laxatives that provide 20 to 25 g/day of raw fiber.
![]() Irritable bowel syndrome (IBS) is one of the most common GI disorders characterized by lower abdominal pain, disturbed defecation, and bloating. Many non-GI manifestations also exist with IBS. Visceral hypersensitivity is a major culprit in the pathophysiology of the disease.
Irritable bowel syndrome (IBS) is one of the most common GI disorders characterized by lower abdominal pain, disturbed defecation, and bloating. Many non-GI manifestations also exist with IBS. Visceral hypersensitivity is a major culprit in the pathophysiology of the disease.
![]() Diarrhea-predominant IBS should be managed by dietary modification and drugs such as loperamide when diet changes alone are insufficient to promote control of symptoms.
Diarrhea-predominant IBS should be managed by dietary modification and drugs such as loperamide when diet changes alone are insufficient to promote control of symptoms.
![]() Several drug classes are involved in the treatment of the pain associated with IBS including tricyclic compounds and the gut-selective calcium channel blockers.
Several drug classes are involved in the treatment of the pain associated with IBS including tricyclic compounds and the gut-selective calcium channel blockers.
DIARRHEA
Diarrhea is a troublesome discomfort that affects most individuals in the United States at some point in their lives and can be thought of as both a symptom and a sign. Usually diarrheal episodes begin abruptly and subside within 1 or 2 days without treatment. This chapter focuses primarily on noninfectious diarrhea, with only minor reference to infectious diarrhea (see Chap. 91 for a discussion of GI infections). Diarrhea is often a symptom of a systemic disease, and not all possible causes of diarrhea are discussed in this chapter. Acute diarrhea is commonly defined as <14 days’ duration, persistent diarrhea as >14 days’ duration, and chronic diarrhea as >30 days’ duration.
To understand diarrhea, one must have a reasonable definition of the condition; unfortunately, the literature is extremely variable on this. Simply put, diarrhea is an increased frequency and decreased consistency of fecal discharge as compared with an individual’s normal bowel pattern. Frequency and consistency are variable within and between individuals. For example, some individuals defecate as often as three times per day, whereas others defecate only two or three times per week. A Western diet usually produces a daily stool weighing between 100 and 300 g, depending on the amount of nonabsorbable materials (mainly carbohydrates) consumed. Patients with serious diarrhea may have a daily stool weight in excess of 300 g; however, a subset of patients experience frequent small, watery passages. Additionally, vegetable fiber-rich diets, such as those consumed in some Eastern cultures (e.g., those in Africa), produce stools weighing more than 300 g/day.
Diarrhea may be associated with a specific disease of the intestines or secondary to a disease outside the intestines. For instance, bacillary dysentery directly affects the gut, whereas diabetes mellitus causes neuropathic diarrheal episodes. Furthermore, diarrhea can be considered as acute or chronic disease. Infectious diarrhea is often acute; diabetic diarrhea is chronic. Congenital disorders in GI ion transport mechanisms are another cause of chronic diarrhea.1 Whether acute or chronic, diarrhea has the same pathophysiologic causes that help in identification of specific treatments.
Epidemiology
The epidemiology of diarrhea varies in developed versus developing countries.2 In the United States, diarrheal illnesses are usually not reported to the Centers for Disease Control and Prevention (CDC) unless associated with an outbreak or an unusual organism or condition. For example, the acquired immune deficiency syndrome (AIDS) has been identified with protracted diarrheal illness. Diarrhea is a major problem in daycare centers and nursing homes, probably because early childhood and senescence plus environmental conditions are risk factors. Although an exact epidemiologic profile in the United States is not available through the CDC or published literature, chronic diarrhea affects approximately 5% of the adult population and ranges from 3% to 20% in children worldwide.3–5 In developing countries, diarrhea is a leading cause of illness and death in children, creating a tremendous economic strain on healthcare costs.
![]() Most cases of acute diarrhea are caused by infections with viruses, bacteria, or protozoa and are generally self-limited.6 Although viruses are more commonly associated with acute gastroenteritis, bacteria are responsible for more cases of acute diarrhea.
Most cases of acute diarrhea are caused by infections with viruses, bacteria, or protozoa and are generally self-limited.6 Although viruses are more commonly associated with acute gastroenteritis, bacteria are responsible for more cases of acute diarrhea.
Evaluation of a noninfectious cause is considered if diarrhea persists and no infectious organism can be identified, or if the patient falls into a high-risk category for metabolic complications with persistent diarrhea. Common causative bacterial organisms include Shigella, Salmonella, Campylobacter, Staphylococcus, and Escherichia coli. Foodborne bacterial infection is a major concern, as several major food poisoning episodes have occurred that were traced to poor sanitary conditions in meat processing plants. Acute viral infections are attributed mostly to the Norwalk and rotavirus groups.
Physiology
In the fasting state, 9 L of fluid enters the proximal small intestine each day. Of this fluid, 2 L is ingested through diet, while the remainder consists of internal secretions. Because of meal content, duodenal chyme is usually hypertonic. When chyme reaches the ileum, the osmolality adjusts to that of plasma, with most dietary fat, carbohydrate, and protein being absorbed. The volume of ileal chyme decreases to about 1 L/day on entering the colon, which is further reduced by colonic absorption to 100 mL daily. If the small intestine water absorption capacity is exceeded, chyme overloads the colon, resulting in diarrhea. In humans, the colon absorptive capacity is about 5 L daily. Colonic fluid transport is critical to water and electrolyte balance.
Absorption from the intestines back into the blood occurs by three mechanisms: active transport, diffusion, and solvent drag. Active transport and diffusion are the mechanisms of sodium transport. Because of the high luminal sodium concentration (142 mEq/L [142 mmol/L]), sodium diffuses from the sodium-rich gut into epithelial cells, where it is actively pumped into the blood and exchanged with chloride to maintain an isoelectric condition across the epithelial membrane.
Hydrogen ions are transported by an indirect mechanism in the upper small intestine. As sodium is absorbed, hydrogen ions are secreted into the gut. Hydrogen ions then combine with bicarbonate ions to form carbonic acid, which then dissociates into carbon dioxide and water. Carbon dioxide readily diffuses into the blood for expiration through the lung. The water remains in the chyme.
Paracellular pathways are major routes of ion movement. As ions, monosaccharides, and amino acids are actively transported, an osmotic pressure is created, drawing water and electrolytes across the intestinal wall. This pathway accounts for significant amounts of ion transport, especially sodium. Sodium plays an important role in stimulating glucose absorption. Glucose and amino acids are actively transported into the blood via a sodium-dependent cotransport mechanism. Cotransport absorption mechanisms of glucose–sodium and amino acid–sodium are extremely important for treating diarrhea.
Gut motility influences absorption and secretion. The amount of time in which luminal content is in contact with the epithelium is under neural and hormonal control. Neurohormonal substances, such as angiotensin, vasopressin, glucocorticoid, aldosterone, and neurotransmitters, also regulate ion transport.
Pathophysiology
![]() Four general pathophysiologic mechanisms disrupt water and electrolyte balance, leading to diarrhea, and are the basis of diagnosis and therapy. These are (a) a change in active ion transport by either decreased sodium absorption or increased chloride secretion; (b) change in intestinal motility; (c) increase in luminal osmolarity; and (d) increase in tissue hydrostatic pressure. These mechanisms have been related to four broad clinical diarrheal groups: secretory, osmotic, exudative, and altered intestinal transit.
Four general pathophysiologic mechanisms disrupt water and electrolyte balance, leading to diarrhea, and are the basis of diagnosis and therapy. These are (a) a change in active ion transport by either decreased sodium absorption or increased chloride secretion; (b) change in intestinal motility; (c) increase in luminal osmolarity; and (d) increase in tissue hydrostatic pressure. These mechanisms have been related to four broad clinical diarrheal groups: secretory, osmotic, exudative, and altered intestinal transit.
Secretory diarrhea occurs when a stimulating substance either increases secretion or decreases absorption of large amounts of water and electrolytes. Substances that cause excess secretion include vasoactive intestinal peptide (VIP) from a pancreatic tumor, unabsorbed dietary fat in steatorrhea, laxatives, hormones (such as secretion), bacterial toxins, and excessive bile salts. Many of these agents stimulate intracellular cyclic adenosine monophosphate and inhibit Na+/K+-adenosine triphosphatase (ATPase), leading to increased secretion. Also, many of these mediators inhibit ion absorption simultaneously. Secretory diarrhea is recognized by large stool volumes (>1 L/day) with normal ionic contents and osmolality approximately equal to plasma. Fasting does not alter the stool volume in these patients.
Poorly absorbed substances retain intestinal fluids, resulting in osmotic diarrhea. This process occurs with malabsorption syndromes, lactose intolerance, administration of divalent ions (e.g., magnesium-containing antacids), or consumption of poorly soluble carbohydrate (e.g., lactulose). As a poorly soluble solute is transported, the gut adjusts the osmolality to that of plasma; in so doing, water and electrolytes flux into the lumen. Clinically, osmotic diarrhea is distinguishable from other types, as it ceases if the patient resorts to a fasting state.
Inflammatory diseases of the GI tract discharge mucus, serum proteins, and blood into the gut. Sometimes bowel movements consist only of mucus, exudate, and blood. Exudative diarrhea affects other absorptive, secretory, or motility functions to account for the large stool volume associated with this disorder.
Altered intestinal motility produces diarrhea by three mechanisms: reduction of contact time in the small intestine, premature emptying of the colon, and bacterial overgrowth. Chyme must be exposed to intestinal epithelium for a sufficient time period to enable normal absorption and secretion processes to occur. If this contact time decreases, diarrhea results. Intestinal resection or bypass surgery and drugs (such as metoclopramide) cause this type of diarrhea. On the other hand, an increased time of exposure allows fecal bacteria overgrowth. A characteristic small intestine diarrheal pattern is rapid, small, coupling bursts of waves. These waves are inefficient, do not allow absorption, and rapidly dump chyme into the colon. Once in the colon, chyme exceeds the colonic capability to absorb water.
Etiologic Examination of the Stool
Stool characteristics are important in assessing the etiology of diarrhea. A description of the frequency, volume, consistency, and color provides diagnostic clues. For instance, diarrhea starting in the small intestine produces a copious, watery or fatty (greasy), and foul-smelling stool; contains undigested food particles; and is usually free from gross blood. Colonic diarrhea appears as small, pasty, and sometimes bloody or mucoid movements. Rectal tenesmus with flatus accompanies large intestinal diarrhea.
Clinical Presentation
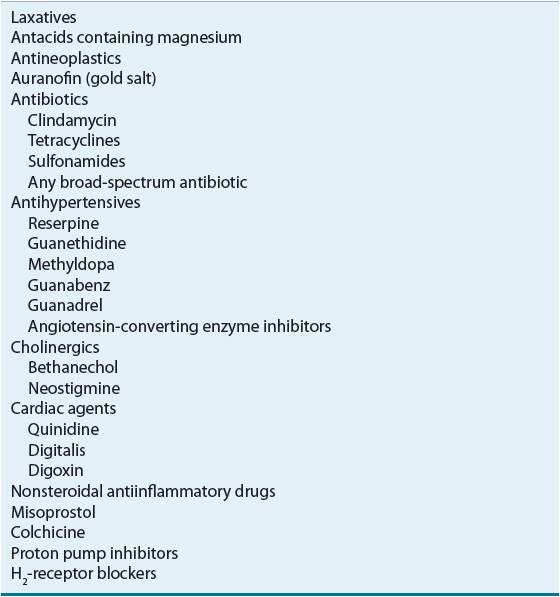
Table 23-1 outlines the clinical presentation of diarrhea, and Table 23-2 shows common drug-induced causes of diarrhea. A medication history is extremely important in identifying drug-induced diarrhea. Many agents, including antibiotics and other drugs, cause diarrhea or, less commonly, pseudomembranous colitis. Self-inflicted laxative abuse for weight loss is popular.
TABLE 23-1 Clinical Presentation of Diarrhea

TABLE 23-2 Drugs Causing Diarrhea
Most acute diarrhea is self-limiting, subsiding within 72 hours. However, infants, young children, the elderly, and debilitated persons are at risk for morbid and mortal events in prolonged or voluminous diarrhea. These groups are at risk for water, electrolyte, and acid–base disturbances, and potentially cardiovascular collapse and death. The prognosis for chronic diarrhea depends on the cause; for example, diarrhea secondary to diabetes mellitus waxes and wanes throughout life.
TREATMENT
Prevention
Acute viral diarrheal illness often occurs in daycare centers and nursing homes. Because person-to-person contact is the mechanism by which viral disease spreads, isolation techniques must be initiated. For bacterial, parasitic, and protozoal infections, strict food handling, sanitation, water, and other environmental hygiene practices can prevent transmission. If diarrhea is secondary to another illness, controlling the primary condition is necessary. Antibiotics and bismuth subsalicylate are advocated to prevent traveler’s diarrhea, in conjunction with treatment of drinking water and caution with consumption of fresh vegetables.7
Desired Outcome
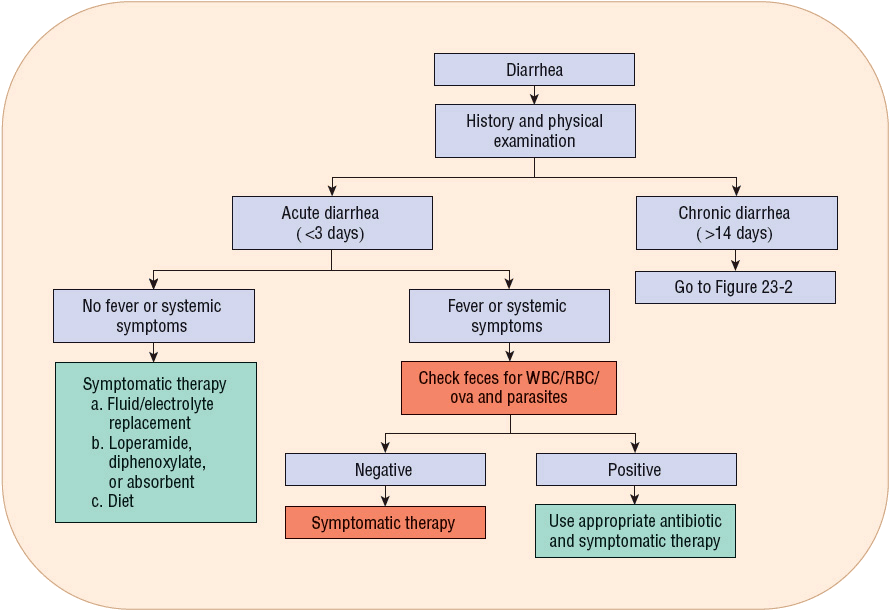
![]() If prevention is unsuccessful and diarrhea occurs, therapeutic goals are to (a) manage the diet; (b) prevent excessive water, electrolyte, and acid–base disturbances; (c) provide symptomatic relief; (d) treat curable causes; and (e) manage secondary disorders causing diarrhea (Figs. 23-1 and 23-2).
If prevention is unsuccessful and diarrhea occurs, therapeutic goals are to (a) manage the diet; (b) prevent excessive water, electrolyte, and acid–base disturbances; (c) provide symptomatic relief; (d) treat curable causes; and (e) manage secondary disorders causing diarrhea (Figs. 23-1 and 23-2).
FIGURE 23-1 Recommendations for treating acute diarrhea. Follow the following steps: (a) Perform a complete history and physical examination. (b) Is the diarrhea acute or chronic? If chronic diarrhea, go to Figure 23-2. (c) If acute diarrhea, check for fever and/or systemic signs and symptoms (i.e., toxic patient). If systemic illness (fever, anorexia, or volume depletion), check for an infectious source. If positive for infectious diarrhea, use appropriate antibiotic/anthelmintic drug and symptomatic therapy. If negative for infectious cause, use only symptomatic treatment. (d) If no systemic findings, then use symptomatic therapy based on severity of volume depletion, oral or parenteral fluid/electrolytes, antidiarrheal agents (see Table 23-4), and diet. (RBC, red blood cells; WBC, white blood cells.)
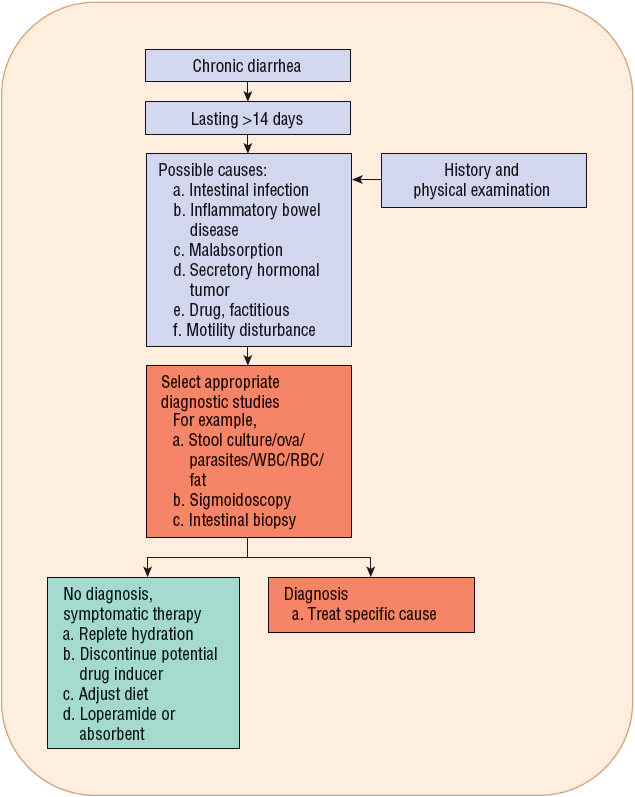
FIGURE 23-2 Recommendations for treating chronic diarrhea. Follow the following steps: (a) Perform a careful history and physical examination. (b) The possible causes of chronic diarrhea are many. These can be classified into intestinal infections (bacterial or protozoal), inflammatory disease (Crohn’s disease or ulcerative colitis), malabsorption (lactose intolerance), secretory hormonal tumor (intestinal carcinoid tumor or vasoactive intestinal peptide-secreting tumor [VIPoma]), drug (antacid), factitious (laxative abuse), or motility disturbance (diabetes mellitus, irritable bowel syndrome, or hyperthyroidism). (c) If the diagnosis is uncertain, selected appropriate diagnostic studies should be ordered. (d) Once diagnosed, treatment is planned for the underlying cause with symptomatic antidiarrheal therapy. (e) If no specific cause can be identified, symptomatic therapy is prescribed. (RBC, red blood cells; WBC, white blood cells.)
Clinicians must clearly understand that diarrhea, like a cough, may be a body defense mechanism for ridding itself of harmful substances or pathogens. The correct therapeutic response is not necessarily to stop diarrhea at all costs.
Nonpharmacologic Management
Dietary management is a first priority in the treatment of diarrhea. Most clinicians recommend discontinuing consumption of solid foods and dairy products for 24 hours. However, fasting is of questionable value, as this treatment modality has not been extensively studied. In osmotic diarrhea, these maneuvers control the problem. If the mechanism is secretory, diarrhea persists. For patients who are experiencing nausea and/or vomiting, a mild, digestible, low-residue diet should be administered for 24 hours. If vomiting is present and uncontrollable with antiemetics (see Chap. 22), nothing is taken by mouth. As bowel movements decrease, a bland diet is begun.
Feeding should continue in children with acute bacterial diarrhea. Fed children have less morbidity and mortality, whether or not they receive oral rehydration fluids. Studies are not available in the elderly or in other high-risk groups to determine the value of continued feeding in bacterial diarrhea.
Water and Electrolytes
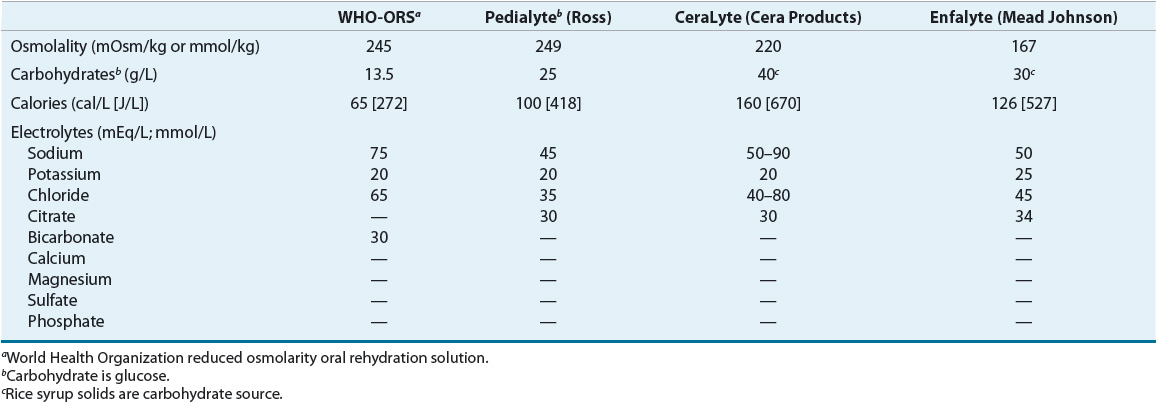
Rehydration and maintenance of water and electrolytes are primary treatment goals until the diarrheal episode ends. If the patient is volume depleted, rehydration should be directed at replacing water and electrolytes to normal body composition. Then water and electrolyte composition are maintained by replacing losses. Many patients will not develop volume depletion and therefore will only require maintenance fluid and electrolyte therapy. Parenteral and enteral routes may be used for supplying water and electrolytes. If vomiting and dehydration are not severe, enteral feeding is the less costly and preferred method. In the United States, many commercial oral rehydration preparations are available (Table 23-3).
TABLE 23-3 Oral Rehydration Solutions
Because of concerns about hypernatremia, physicians continue to hospitalize patients and use IV fluids to correct fluid and electrolyte deficits in severe dehydration. Oral solutions are strongly recommended.8–10 In developing countries, the World Health Organization oral rehydration solution (WHO-ORS) saves the lives of millions of children annually.
During diarrhea, the small intestine retains its ability to actively transport monosaccharides such as glucose. Glucose actively carries sodium with water and other electrolytes. The WHO now recommends an ORS with a lower osmolarity, sodium content, and glucose load (see Table 23-3).11 A separate oral supplement of zinc 20 mg daily for 14 days in addition to ORS significantly reduces the severity and duration of acute diarrhea in developing countries.8,12 ORS is a lifesaving treatment for millions afflicted in developing countries. Acceptance in developed countries is less enthusiastic; however, the advantage of this product in reducing hospitalizations may prove its use as a cost-effective alternative, saving millions of dollars in healthcare expenditures.
Pharmacologic Therapy
Various drugs have been used to treat diarrheal attacks (Table 23-4), including antimotility agents, adsorbents, antisecretory compounds, antibiotics, enzymes, and intestinal microflora. Usually these drugs are not curative but palliative.
TABLE 23-4 Selected Antidiarrheal Preparations
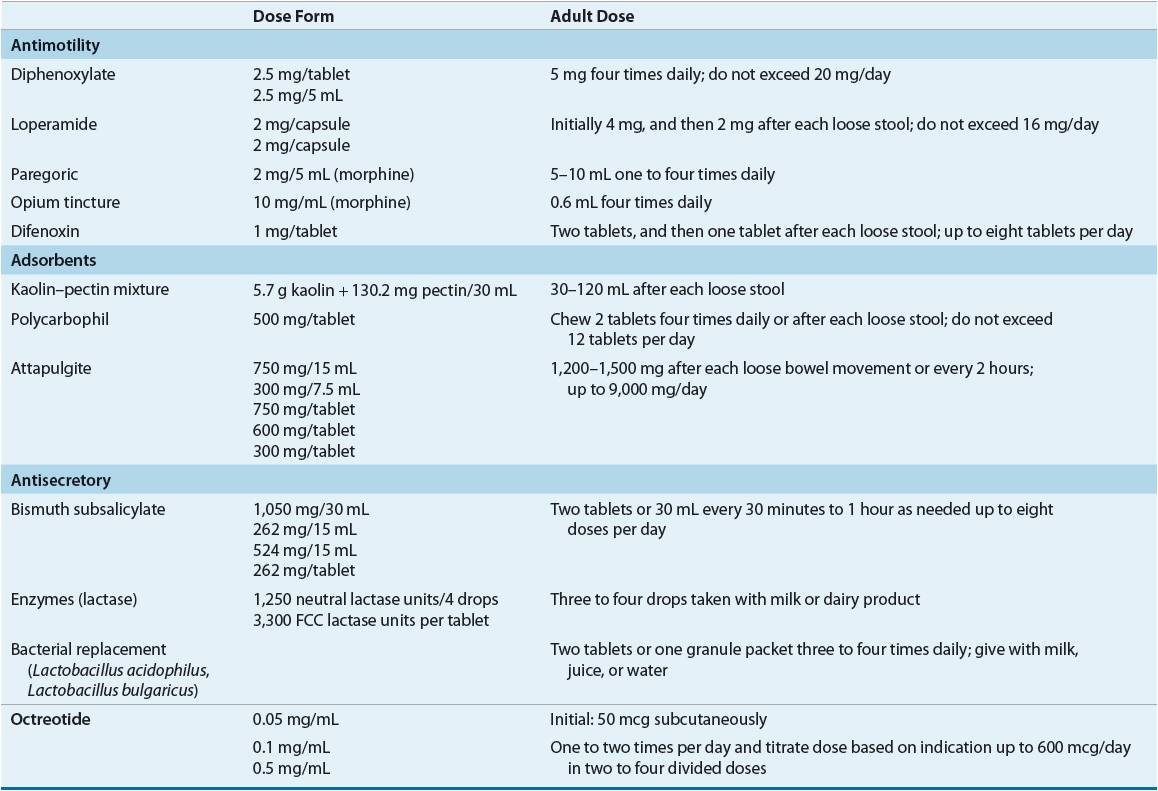
Opiates and Their Derivatives
Opiates and opioid derivatives (a) delay the transit of intraluminal contents or (b) increase gut capacity, prolonging contact and absorption. Enkephalins, which are endogenous opioid substances, regulate fluid movement across the mucosa by stimulating absorptive processes. Limitations to the use of opiates include an addiction potential (a real concern with long-term use) and worsening of diarrhea in selected infectious diarrhea.
Most opiates act through peripheral and central mechanisms with the exception of loperamide, which acts only peripherally. Loperamide is antisecretory; it inhibits the calcium-binding protein calmodulin, controlling chloride secretion. Loperamide, available as 2 mg capsules or 1 mg/5 mL solution (both are nonprescription products), is suggested for managing acute and chronic diarrhea. The usual adult dose is initially 4 mg orally, followed by 2 mg after each loose stool, up to 16 mg/day. Used correctly, this agent has rare side effects, such as dizziness and constipation. If the diarrhea is concurrent with a high fever or bloody stool, the patient should be referred to a physician. Also, diarrhea lasting 48 hours beyond initiating loperamide warrants medical attention. Loperamide can also be used in traveler’s diarrhea. It is comparable to bismuth subsalicylate for treatment of this disorder.7
Diphenoxylate is available as a 2.5 mg tablet and as a 2.5 mg/5 mL solution. A small amount of atropine (0.025 mg) is included in the product to discourage abuse. In adults, when taken as 2.5 to 5 mg three or four times daily, not to exceed a 20 mg total daily dose, diphenoxylate is rarely toxic. Some patients may complain of atropinism (blurred vision, dry mouth, and urinary hesitancy). Like loperamide, it should not be used in patients who are at risk of bacterial enteritis with E. coli, Shigella, or Salmonella.
Difenoxin, a diphenoxylate derivative also chemically related to meperidine, is also combined with atropine and has the same uses, precautions, and side effects. Marketed as a 1 mg tablet, the adult dosage is 2 mg initially, followed by 1 mg after each loose stool, not to exceed 8 mg/day.
Paregoric, camphorated tincture of opium, is marketed as a 2 mg/5 mL solution and is indicated for managing both acute and chronic diarrhea. It is not widely prescribed today because of its abuse potential.
Clinical Controversy…
Adsorbents
Adsorbents are used for symptomatic relief. These products, many not requiring a prescription, are nontoxic, but their effectiveness remains unproven. Adsorbents are nonspecific in their action; they adsorb nutrients, toxins, drugs, and digestive juices. Polycarbophil absorbs 60 times its weight in water and can be used to treat both diarrhea and constipation. It is a nonprescription product and is sold as a 500 mg chewable tablet. This hydrophilic, nonabsorbable product is safe and may be taken four times daily, up to 6 g/day in adults. See Table 23-4 for selected antidiarrheal preparations.
Antisecretory Agents
Bismuth subsalicylate appears to have antisecretory, antiinflammatory, and antibacterial effects. As a nonprescription product, it is marketed for indigestion, relieving abdominal cramps, and controlling diarrhea, including traveler’s diarrhea. Bismuth subsalicylate dosage strengths are a 262 mg chewable tablet, 262 mg/5 mL liquid, and 524 mg/15 mL liquid. The usual adult dose is two tablets or 30 mL every 30 minutes to 1 hour up to eight doses per day.
![]() Bismuth subsalicylate contains multiple components that might be toxic if given excessively to prevent or treat diarrhea. For instance, an active ingredient is salicylate, which may interact with anticoagulants or may produce salicylism (tinnitus, nausea, and vomiting). Bismuth reduces tetracycline absorption and may interfere with select GI radiographic studies. Patients may complain of a darkening of the tongue and stools with repeat administration. Salicylate can induce gout attacks in susceptible individuals.
Bismuth subsalicylate contains multiple components that might be toxic if given excessively to prevent or treat diarrhea. For instance, an active ingredient is salicylate, which may interact with anticoagulants or may produce salicylism (tinnitus, nausea, and vomiting). Bismuth reduces tetracycline absorption and may interfere with select GI radiographic studies. Patients may complain of a darkening of the tongue and stools with repeat administration. Salicylate can induce gout attacks in susceptible individuals.
Bismuth subsalicylate suspension has been evaluated in the treatment of secretory diarrhea of infectious etiology as well. In a dose of 30 mL every 30 minutes for eight doses, unformed stools decrease in the first 24 hours. Bismuth subsalicylate may also be effective in preventing traveler’s diarrhea.
Octreotide, a synthetic octapeptide analog of endogenous somatostatin, is effective for the symptomatic treatment of carcinoid tumors and other peptide-secreting tumors, dumping syndrome, and chemotherapy-induced diarrhea.13 It has had limited success in patients with AIDS-associated diarrhea and short-bowel syndrome, does not appear to have an advantage over various opiate derivatives in the treatment of chronic idiopathic diarrhea, and has the disadvantage of being administered by injection.14 Metastatic intestinal carcinoid tumors secrete excessive amounts of vasoactive substances, including histamine, bradykinin, serotonin (5-HT), and prostaglandins. Primary carcinoid tumors occur throughout the GI tract, with most in the ileum. Predominant signs and symptoms experienced by patients with these tumors are attributable to excessive concentrations of 5-hydroxytryptophan and 5-HT. The totality of their clinical effects is termed the carcinoid syndrome. Some patients have a violent, watery diarrhea with abdominal cramping. Initially, diarrhea might be managed with various agents such as codeine, diphenoxylate, cyproheptadine, methysergide, phenoxybenzamine, or methyldopa. But octreotide is now considered first-line therapy for carcinoid syndrome.
Octreotide blocks the release of 5-HT and many other active peptides and has been effective in controlling diarrhea and flushing. It is reported to have direct inhibitory effects on intestinal secretion and stimulatory effects on intestinal absorption. Non–gastrin-secreting adenomas of the pancreas are tumors associated with profuse watery diarrhea. This condition has been referred to as Verner-Morrison syndrome, WDHA (watery diarrhea, hypokalemia, and achlorhydria) syndrome, pancreatic cholera, watery diarrhea syndrome, and vasoactive intestinal peptide-secreting tumor (VIPoma). Excessive secretion of VIP from a retroperitoneal or pancreatic tumor produces most of the clinical features. Surgical tumor dissection is the treatment of choice. In nonsurgical candidates, the profuse watery diarrhea and other symptoms commonly encountered are managed with octreotide.
The dose of octreotide varies with the indication, disease severity, and patient response.13 For managing diarrhea and flushing associated with carcinoid tumors in adults, the initial dosage range is 100 to 600 mcg/day in two to four divided doses subcutaneously for 2 weeks. For controlling secretory diarrhea of VIPomas, the dosage range is 200 to 300 mcg/day in two to four divided doses for 2 weeks. Some patients may require higher doses for symptomatic control. Patients responding to these initial doses may be switched to Sandostatin LAR Depot, a long-acting octreotide formulation. This product consists of microspheres containing the drug. Initial doses consist of 20 mg given intramuscularly intragluteally at 4-week intervals for 2 months. It is recommended that during the first 2 weeks of therapy the short-acting formulation also be administered subcutaneously. At the end of 2 months, patients with good symptom control may have the dose reduced to 10 mg every 4 weeks, while those without sufficient symptom control may have the dose increased to 30 mg every 4 weeks. For patients experiencing recurrence of symptoms on the 10 mg dose, dosage adjustment to 20 mg should be made. It is not uncommon for patients with carcinoid tumors or VIPomas to experience periodic exacerbation of symptoms. Subcutaneous octreotide for several days should be reinstituted in these individuals. In so-called carcinoid crisis, octreotide is given as an IV infusion at 50 mcg/h for 8 to 24 hours.
Because octreotide inhibits many other GI hormones, it has a variety of intestinal side effects. With prolonged use, gallbladder and biliary tract complications such as cholelithiasis have been reported. Approximately 5% to 10% of patients complain of nausea, diarrhea, and abdominal pain. Local injection pain occurs with about an 8% incidence. With high doses, octreotide may reduce dietary fat absorption, leading to steatorrhea.
Two other somatostatin analogs, lanreotide and vapreotide, have been studied.14,15 Lanreotide is approved for use in the United States for acromegaly. The starting dose is 90 mg subcutaneously every 4 weeks for 3 months, and then the dose is adjusted based on growth hormone and insulin-like growth factor levels.16 Vapreotide is an orphan drug that is indicated for pancreatic and GI fistulas as well as esophageal variceal bleeding.
Miscellaneous Products
Probiotics are microorganisms that have been used for many years to replace colonic microflora. This supposedly restores normal intestinal function and suppresses the growth of pathogenic microorganisms. Saccharomyces boulardii, Lactobacillus GG, and Lactobacillus acidophilus decrease the duration of infectious and antibiotic-induced diarrhea in adults and children.17 A combination probiotic product, VSL#3 (which contains multiple strains of lactobacilli and bifidobacteria) may have benefit in preventing radiation-induced diarrhea when given three times a day.18 A meta-analysis suggests that probiotics may prevent antibiotic-associated diarrhea (AAD).19 The dosage of probiotic preparations varies depending on the brand used. Intestinal flatus is the primary patient complaint experienced with this modality.
Anticholinergic drugs such as atropine block vagal tone and prolong gut transit time. Drugs with anticholinergic properties are present in many nonprescription products. Their value in controlling diarrhea is questionable and limited because of side effects. Angle-closure glaucoma, selected heart diseases, and obstructive uropathies are relative contraindications to the use of anticholinergic agents.
Lactase enzyme products are helpful for patients who are experiencing diarrhea secondary to lactose intolerance. Lactase is required for carbohydrate digestion. When a patient lacks this enzyme, eating dairy products causes an osmotic diarrhea. Several products are available for use each time a dairy product, especially milk or ice cream, is consumed.