, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Diabetic nephropathy is a clinical syndrome in a patient with diabetes mellitus that is characterized by persistent albuminuria, worsening proteinuria, hypertension, and progressive renal failure [1–3]. Approximately a third of patients with type 1 insulin-dependent diabetes mellitus (IDDM) and type 2 non-insulin-dependent diabetes mellitus (NIDDM) develop diabetic nephropathy [2]. The pathologic hallmark of diabetic nephropathy is diabetic glomerulosclerosis that results from a progressive increase in extracellular matrix in the glomerular mesangium and glomerular basement membranes [4]. Diabetic glomerulosclerosis is the leading cause of end-stage renal disease in the United States, Europe, and Japan [1].
Introduction/Clinical Setting
Diabetic nephropathy is a clinical syndrome in a patient with diabetes mellitus that is characterized by persistent albuminuria, worsening proteinuria, hypertension, and progressive renal failure [1–3]. Approximately a third of patients with type 1 insulin-dependent diabetes mellitus (IDDM) and type 2 non-insulin-dependent diabetes mellitus (NIDDM) develop diabetic nephropathy [2]. The pathologic hallmark of diabetic nephropathy is diabetic glomerulosclerosis that results from a progressive increase in extracellular matrix in the glomerular mesangium and glomerular basement membranes [4]. Diabetic glomerulosclerosis is the leading cause of end-stage renal disease in the United States, Europe, and Japan [1].
Pathologic Findings
Light Microscopy
Diabetic nephropathy causes pathologic abnormalities in all of the major structural compartments of the kidney, including the glomeruli, extra-glomerular vessels, interstitium, and tubules [3–15].
The earliest glomerular change is enlargement (hypertrophy, hyperplasia, glomerulomegaly), which corresponds to the early clinical phase of elevated glomerular filtration rate. By the time albuminuria is detectable, there is generalized thickening of glomerular basement membranes (GBMs) and an increase in mesangial matrix material. In the earliest phase, morphometry is required to detect these changes, but eventually the GBM thickening and mesangial expansion is so pronounced that it can be readily discerned by routine light microscopy, especially if a special stain that accentuates collagenous structures is used [e.g., periodic acid–Schiff (PAS), Jones silver, Masson trichrome]. Mild mesangial hypercellularity occasionally accompanies the matrix expansion; thus, care must be taken not to misdiagnose early diabetic glomerulosclerosis as mesangioproliferative glomerulonephritis.
Overt glomerular mesangial matrix expansion (glomerulosclerosis) manifests as diffuse mesangial matrix expansion or nodular mesangial matrix expansion or, most often, a combination of both (Figs. 12.1, 12.2, 12.3, 12.4, and 12.5). Glomerular basement membrane thickening usually accompanies the mesangial matrix expansion, but it may be somewhat discordant in severity [6]. The designations diffuse versus nodular glomerulosclerosis are primarily of descriptive value in the biopsy report and have no value in the diagnosis because the distinctions do not have clinical significance.
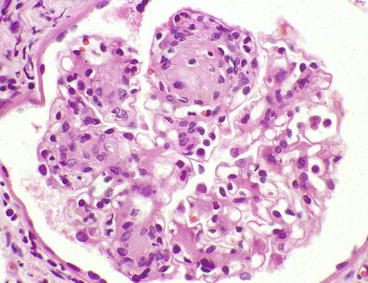
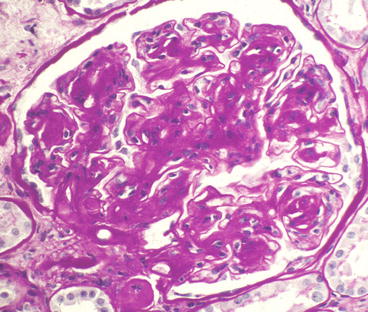
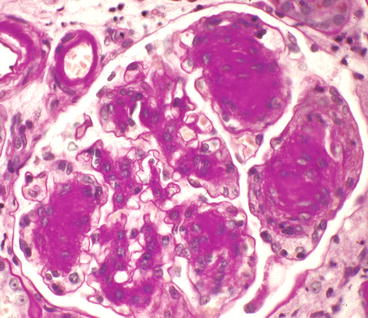
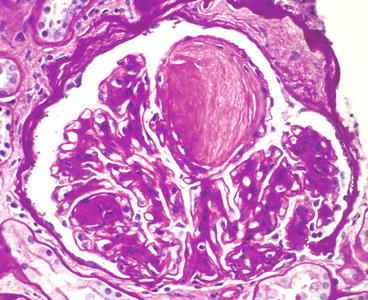
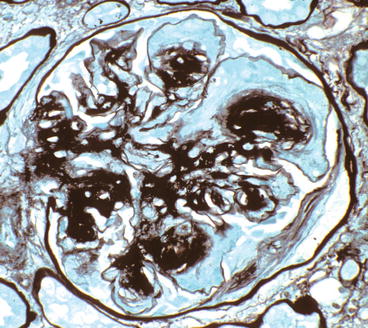

Fig. 12.1
Glomerulus from patient with diabetic glomerulosclerosis showing segmental mesangial matrix expansion and hypercellularity that is most pronounced on the left. The upper pole has a Kimmelstiel–Wilson (K–W) nodule [hematoxylin and eosin (H&E) stain]

Fig. 12.2
Glomerulus from patient with diabetic glomerulosclerosis showing relatively diffuse mesangial matrix expansion, although there is slight nodularity in some segments [periodic acid–Schiff (PAS) stain]

Fig. 12.3
Glomerulus from patient with diabetic glomerulosclerosis showing multiple K–W nodules (PAS stain). The afferent and efferent arterioles in the upper left corner both have PAS-positive hyalinosis

Fig. 12.4
Glomerulus from patient with diabetic glomerulosclerosis showing a large K–W nodule with vague lamination (PAS stain)

Fig. 12.5
Glomerulus from patient with diabetic glomerulosclerosis showing extensive capillary aneurysm formation as a result of mesangiolysis that has released the GBM from the mesangium (silver stain)
Diffuse diabetic glomerulosclerosis is less specific for diabetic glomerulosclerosis than nodular diabetic glomerulosclerosis. Especially if the clinical presence of diabetes is not known and there is accompanying mesangial hypercellularity, the light microscopic changes can be mistaken for a mesangioproliferative glomerulonephritis. Careful examination may reveal early mesangial nodules, which will suggest the correct diagnosis.
The nodular lesions of diabetic glomerulosclerosis were first described by Kimmelstiel and Wilson [5] and thus are called Kimmelstiel–Wilson (K–W) nodules. The nodules begin in the heart of the mesangial region of a segment. As the nodule of matrix accrues, there may be increased numbers of mesangial cells, especially at its leading edges (Fig. 12.1). The nodules often are focal and segmental, although occasional specimens have rather diffuse global nodularity. The nodules have the same tinctorial properties as normal mesangial matrix and thus are PAS and silver positive (Figs. 12.3, 12.4, and 12.5). The matrix at the center of the nodules may be homogeneous or laminated (Fig. 12.4). K–W nodules may have a corona of capillary aneurysms that are formed as a result of mesangiolysis, which disrupts the attachment points of the GBM to the mesangium (Fig. 12.5).
Glomerular hyalinosis is common in diabetic glomerulosclerosis. These hyaline lesions putatively result from insudation or exudation of plasma proteins from vessels followed by entrapment in matrix. The hyalinosis can occur anywhere in the tuft, but there are two characteristic patterns: hyaline caps and capsular drops. The hyaline caps are produced when the hyalinosis forms arcs at the periphery of segments, sometimes appearing to fill the capillary aneurysms. Capsular drops are spherical accumulations of hyaline material adjacent to or within Bowman’s capsule.
Crescent formation is identified in <5 % of specimens with diabetic glomerulosclerosis (Fig. 12.6). When crescents are observed, one should consider the possibility of a concurrent glomerulonephritis that is more often associated with crescents, such as antineutrophil cytoplasmic antibodies (ANCA) disease or anti-GBM disease. However, small numbers of crescents may result from diabetic injury alone, possibly as a consequence of rupture of peripheral capillary aneurysms.
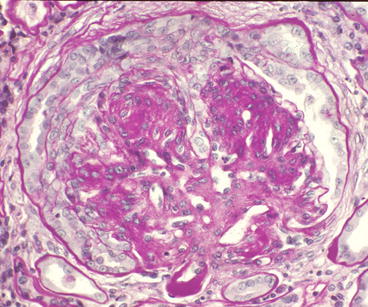

Fig. 12.6
Glomerulus from patient with diabetic glomerulosclerosis showing cellular crescent formation (PAS stain). No other glomerular disease was identified. Note the hyalinosis of the efferent arteriole
Diabetic glomerulosclerosis is caused by both type 1 (IDDM) and type 2 (NIDDM). The latter is more heterogeneous in appearance [7, 10, 13, 15], in part because it often is altered by concurrent hypertensive and aging changes. At a comparable stage of diabetic nephropathy, the glomerular lesions in type 2 diabetes tend to be less severe than those in type 1 [9, 15].
Arteriolosclerosis and arteriosclerosis are typical accompaniments to diabetic glomerulosclerosis. Arteriolar hyalinosis at the glomerular hilum is ubiquitous with diabetic glomerulosclerosis and typically affects both the afferent and efferent arterioles [4, 12]. Hypertensive hyaline arteriolar sclerosis affects the afferent but not efferent arteriole.
The earliest tubular change is thickening of tubular basement membranes (TBMs) that is analogous to the GBM thickening (Fig. 12.7) [4]. With progressive chronic disease, tubules become atrophic and the interstitium develops fibrosis and chronic inflammation. Except for the marked TBM thickening, these chronic tubulointerstitial changes resemble those seen with any form of progressive glomerular disease.
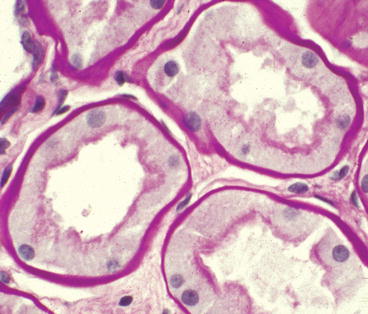

Fig. 12.7
Proximal tubules from patient with diabetic glomerulosclerosis showing markedly thickened tubular basement membranes even though there is no tubular atrophy or interstitial fibrosis (PAS stain)
Immunofluorescence Microscopy
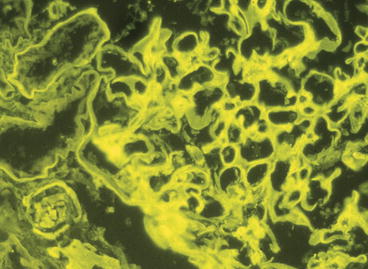
Typical diabetic glomerulosclerosis usually can be diagnosed with reasonable accuracy from the immunofluorescence microscopy findings alone. The characteristic feature is linear staining of GBMs with antisera specific for immunoglobulin G (IgG) and other plasma proteins, although the staining for IgG is usually brightest (Fig. 12.8). Kappa light chain staining usually is brighter than lambda light chain staining. Immunofluorescence microscopy is useful for ruling out other glomerular diseases that can mimic diabetic glomerulosclerosis by light microscopy, such as monoclonal immunoglobulin deposition disease, membranoproliferative glomerulonephritis, fibrillary glomerulonephritis, and amyloidosis. Bowman’s capsule and TBMs also often show linear staining.