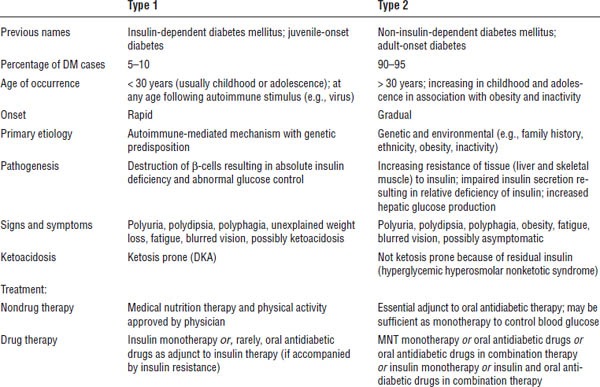
Table 17-1. Comparison of Type 1 and Type 2 Diabetes Mellitus
Clinical Presentation
Classic signs and symptoms include polydipsia, polyuria, and polyphagia. Other common findings include fatigue, blurred vision, and frequent infections.
Type 1 diabetes
Onset is rapid. In addition to the signs above, it may also include unexplained weight loss. Patients may be ketonuric or experience ketoacidosis. They may experience a “honeymoon” period, a phase of erratic insulin secretion lasting months to a year during destruction of β-cells.
Type 2 diabetes
Onset is gradual and progressive. Patients may be asymptomatic or experience classic signs and symptoms. Most are obese or have a history of obesity. Patients may present with existing microvascular or macrovascular chronic complications, or both.
Pathophysiology and Etiology
Type 1 diabetes
■ β-cell destruction leads to absolute insulin deficiency.
■ Patients are prone to ketoacidosis.
■ Peak onset occurs at the time of puberty but may occur at any age.
Type 2 diabetes
■ Insulin resistance and progressive β-cell dysfunction occur.
■ It often involves a strong genetic predisposition.
■ It is associated with environmental factors such as excessive caloric intake, decreased activity, weight gain, and obesity.
■ Insulin resistance may be present years before the onset of DM.
■ Initially normal glucose levels are maintained by increased insulin secretion by β-cells.
■ Increasing insulin resistance or a failure of β-cells to maintain insulin secretion eventually leads to the development of DM.
■ Insulin resistance is influenced by age, ethnicity, physical activity, medications, and weight.
■ It is usually diagnosed in adulthood but can occur at any age with recent increases in diagnosis in children and adolescents.
■ Incidence is higher among certain ethnic populations (American Indians/Alaska Natives, non-Hispanic blacks, Hispanic/Latinos, and non-Hispanic whites).
Diagnostic Criteria
Box 17-1 shows the criteria for the diagnosis of DM.
Type 1 and type 2 DM
Diagnosis can be made on the basis of an A1C, an FPG test, a random plasma glucose test, or an oral glucose tolerance test (OGTT). The A1C may be more convenient because fasting is not required, and daily changes are possible during stress or illness in an FPG. These advantages may be balanced by greater cost and limited availability of A1C testing in certain regions of the world. The A1C may not be as accurate with certain forms of anemia (e.g., iron deficiency, hemolysis) and hemoglobinopathies (e.g., sickle cell trait).
Gestational diabetes mellitus
There are two methods to test for gestational diabetes. Women not known to have DM prior to pregnancy should undergo testing between 24 and 28 weeks of gestation.
Box 17-1. Criteria for the Diagnosis of Diabetes Mellitus
Hemoglobin A1C ≥ 6.5%a
OR
Fasting plasma glucose (FPG) ≥ 126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 hours.a
OR
Two-hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT) using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.a
OR
In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81–S90.
a. In the absence of unequivocal hyperglycemia, the first three criteria should be confirmed by repeat testing of the same test.
The one-step approach includes administration of a 75 g OGTT with glucose measurements fasting (overnight fast at least 8 hours), 1 hour and 2 hours post-glucose load.
The diagnosis of GDM is made when any of the following glucose values is exceeded:
■ Fasting: ≥ 92 mg/dL (5.1 mmol/L)
■ 1 hour: ≥ 180 mg/dL (10 mmol/L)
■ 2 hours: ≥ 153 mg/dL (8.5 mmol/L)
The two-step approach includes administration of a nonfasting 50 g glucose challenge test with glucose assessment 1 hour after glucose administration. If the plasma glucose value at 1 hour is ≥ 140 mg/dL, then the patient proceeds to a 3-hour 100g OGTT. The diagnosis of GDM is made when the plasma glucose is ≥ 140 mg/dL 3 hours after the glucose load.
Treatment Principles and Goals
■ To achieve and maintain glycemic control. Treatment goals should be individualized on the basis of patient characteristic such as duration of DM, age, life expectancy, comorbid conditions, known CVD, presence of microvascular complications, hypoglycemic unawareness, and other individual patient considerations. In general, the glycemic goals include A1C < 7%, preprandial capillary plasma glucose 70–130 mg/dL, and 1–2 hour postprandial capillary plasma glucose < 180 mg/dL. Individual patient goals may be more or less stringent than these parameters.
■ To attain recommended blood pressure and lipid goals (see chapters 12 and 16 for disease goals and management)
■ To modify lifestyle to promote general health and achieve weight management goals
■ To prevent or slow progression of chronic complications
■ To prevent or resolve acute complications
■ To achieve an acceptable quality of life and satisfaction with care
Prevention of complications
The following activities can prevent complications from arising:
■ Cessation of tobacco use
■ Aspirin or anti platelet therapy
• Consider aspirin (75–162 mg/day), or clopidogrel if patient is allergic to aspirin, as secondary prevention for diabetics with a history of CVD.
• Use as primary prevention with 10-year cardiovascular risk > 10%. It should not be recommended for primary prevention for diabetics with low CVD risk (10-year risk < 5%) because of potential adverse effects. If 10-year risk is between 5% and 10%, clinical judgment should be used regarding antiplatelet therapy.
■ Immunization
• Annual influenza vaccine if patient is 6 months of age or older and no contraindications
• At least one lifetime pneumococcal polysaccharide vaccine for patients ≥ 2 years of age with no contraindications
• One-time pneumococcal polysaccharide revaccination if the patient is > 65 years of age and was previously immunized > 5 years ago
• Hepatitis B vaccination for previously unvaccinated adults 19–59 years of age
■ Foot care: self-inspection daily, visual inspection at each office visit, and an annual comprehensive exam (i.e., foot pulses, testing for loss of protective sensation) by a medical provider
■ Skin care: self-inspection and care daily
■ Dental care: annual examination
■ Eye care: annual dilated eye examination starting at the time of diagnosis of type 2 DM or 5 years after the diagnosis of type 1 DM
■ Nephropathy screening: annual serum creatinine for estimated glomerular filtration rate (eGFR); annual evaluation of urine albumin excretion starting at the time of diagnosis of type 2 DM or 5 years after the diagnosis of type 1 DM
Complications of diabetes
Macrovascular disease
■ Coronary atherosclerosis: Rate is two to four times higher in adults with DM.
■ Cerebrovascular atherosclerosis: Stroke risk is two to four times higher among people with DM.
■ Peripheral vascular disease: Insufficient circulation impairs healing and increases risk of amputation.
Microvascular disease
■ Retinopathy
• DM is the leading cause of new cases of blindness in adults 20–74 years of age.
• Retinopathy may develop without symptoms; annual dilated eye examination is recommended for detection.
• Treatment includes glycemic control, blood pressure control, and laser photocoagulation.
• It occurs in 20–40% of diabetics and is the leading cause of end-stage renal disease.
• Treatment includes glycemic and blood pressure control; angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers should be used except during pregnancy.
■ Distal symmetric polyneuropathy
• Sensorimotor nervous system dysfunction occurs.
• Pain and diminished sensation occur, with potential for poor detection of trauma; polyneuropathy increases risk for ulceration and infection.
• See chapter 27 (Pain Management and Migraines) for information about treatment of distal symmetric polyneuropathy.
■ Autonomic neuropathy
• Gastrointestinal (GI) effects include gastroparesis, constipation, and diarrhea.
• Genitourinary effects include neurogenic bladder and sexual dysfunction in men.
• Cardiovascular effects include orthostatic hypotension and resting tachycardia.
■ Diabetic foot problems
• DM accounts for > 60% of nontraumatic amputations in the United States.
• Prevention, early detection with regular foot exams, and prompt treatment of lesions are essential to avoid complications.
Acute complications
■ Hypoglycemia
• Definition is plasma glucose < 70 mg/dL.
• Glucose (15–20 g) is the preferred treatment.
• Other sources to provide 15–20 g of carbohydrates can be used (e.g., 4 oz fruit juice, 4 oz regular soda).
• Treatment effects should be seen in approximately 15 minutes.
• Symptoms can range from mild (tremor, palpitations, sweating) to severe (unresponsiveness, unconsciousness, or convulsions).
• Severe hypoglycemia may require assistance from another individual for treatment with subcutaneous glucagon injection or intravenous glucose.
■ Diabetic ketoacidosis (DKA)
• In type 1 DM, DKA is a medical emergency caused by absolute or relative insulin deficiency.
• Omission of insulin, major stress, infection, or trauma may precipitate DKA.
• DKA is characterized by glucose > 250 mg/dL, elevated ketones, arterial pH < 7.2, and plasma bicarbonate < 15 mEq/L.
• Ketone bodies are formed in excess because of fatty acid metabolism in the liver, leading to ketonuria and ketonemia and ultimately diabetic ketoacidosis.
• Kussmaul respirations (deep and rapid) are characteristic in an attempt to compensate for metabolic acidosis.
• DKA requires prompt intervention with insulin, fluids, and electrolytes to prevent coma and death.
■ Hyperglycemic hyperosmolar state
• A complication of type 2 DM, this is also known as hyperglycemic hyperosmolar nonketotic coma.
• It is characterized by elevated plasma glucose (typically > 500 mg/dL), dehydration, and hyperosmolarity in the absence of significant ketoacidosis.
• It may be triggered by infection or other stressors such as stroke or myocardial infarction.
• Treatment includes fluid and electrolyte replacement as well as insulin.
17-4. Drug Therapy
Oral Medications for DM Treatment
Biguanides (Table 17-2)
Mechanism of action
The primary mechanism is seen through decreased hepatic gluconeogenesis as well as improved glucose utilization and uptake in peripheral tissues and decreased intestinal absorption of glucose.
Clinical considerations
■ Biguanides are considered the first choice when beginning drug treatment in newly diagnosed type 2 DM unless they are contraindicated or the patient is not able to tolerate them.
■ They pose minimal risk of hypoglycemia unless combined with secretagogues or insulin.
■ They may decrease weight up to 5 kg.
■ They decrease triglycerides, decrease low-density lipoprotein (LDL), and increase high-density lipoprotein (HDL).
■ GI symptoms (nausea, vomiting, bloating, flatulence, anorexia, and diarrhea) are the most common adverse effects.
• Take doses with or after meals to reduce GI symptoms.
• GI symptoms are transient and improve in most patients over time.
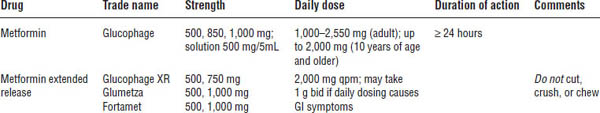
• Titrate the dose up slowly to minimize GI symptoms: 500 mg daily with the largest meal × 1 week; then increase to 500 mg twice daily with the two largest meals × 1 week; then increase to 1 g (two 500 mg tablets) with the largest meal and 500 mg with the second-largest meal × 1 week; and then increase to 1 g twice daily with the two largest meals of the day.
■ Biguanides interfere with vitamin B12 absorption. B12 deficiency may cause symptoms of peripheral neuropathy that are similar to symptoms of diabetes-related peripheral neuropathy. B12 deficiency may also cause macrocytic anemia. Monitor vitamin B12 concentrations annually.
■ They may require as much as 8 weeks of therapy before effectiveness can be assessed.
■ Biguanides can be used in patients with type 2 diabetes who are pregnant, but they do not have an indication for use approved by the U.S. Food and Drug Administration (FDA). They are considered safe to use during breast-feeding.
■ They are indicated for the treatment of type 2 DM in children 10 years of age and older.
■ They may decrease the progression to DM from IGT and IFG (prediabetes).
■ They have positive cardiovascular benefits when used in obese patients with DM.
A1C reduction
A reduction of 1–2% is expected.
Cautions and contraindications
Most cautions and contraindications are related to the ability of biguanides to increase the risk of lactic acidosis with metformin (less than one case per 100,000 treated patients).
Contraindications
■ Renal insufficiency (serum creatinine [SCr], or SCr ≥ 1.4 females and SCr ≥ 1.5 males) is a contraindication; recent studies have suggested that metformin is safe unless the eGFR falls to < 30 mL/min.
■ Hepatic dysfunction is a contraindication.
■ Excessive alcohol use (binge or chronic use of more than two drinks per day or at one sitting) is a contraindication.
■ Medication should be avoided in patients with unstable symptomatic congestive heart failure (New York Heart Association classifications III and IV).
Cautions
■ Medication should be discontinued in situations of increased risk for lactic acidosis, including acute myocardial infarction, congestive heart failure exacerbation, severe respiratory disease, shock, and septicemia.
■ Medication should be discontinued before procedures that require iodinated contrast media and major surgeries. It can be resumed 2–3 days after renal function has returned to baseline and remains stable.
Secretagogues (Table 17-3)
Mechanism of action
The primary mechanism of secretagogues is to cause a reduction in blood glucose by stimulating the release of insulin from the pancreas. This mechanism may in turn cause a decrease in hepatic gluconeogenesis and a slight decrease in insulin resistance at the muscle level. Effectiveness depends on pancreatic β-cell function.
Clinical and counseling considerations
■ Medication should be taken before meals: sulfonylureas, once or twice daily; meglitinides, before each meal.
■ Medication causes a 1–2 kg weight gain.
■ A positive risk of hypoglycemia exists, which is greater with sulfonylureas than with meglitinides, because of differences in duration of action.
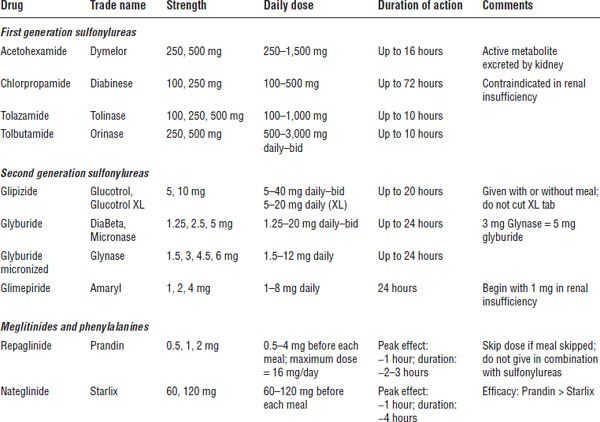
■ Secretagogues are typically not indicated during pregnancy, when breast-feeding, or in children. However, glyburide can be used during pregnancy and breast-feeding.
■ A fast-acting oral carbohydrate should be carried for emergency use.
A1C reduction
■ 1–2% (sulfonylureas)
■ 0.5–2% (meglitinides)
Cautions and contraindications
■ Use with caution in elderly patients; do not use chlorpropamide.
■ Use with caution in cases of renal and hepatic insufficiency; glipizide and glimepiride are safer.
■ Avoid in patients with significant alcohol use.
■ Drug interactions (worse with first-generation sulfonylureas) may cause increased risk of hypoglycemia: anticoagulants, fluconazole, salicylates, gemfibrozil, sulfonamides, tricyclic antidepressants, digoxin.
■ Use is contraindicated in patients with DKA, severe infection, surgery, or trauma.
■ Patients should wear medical identification.
■ Store drug in a cool, dry place (not the bathroom or kitchen).
■ Syndrome of inappropriate antidiuretic hormone secretion, disulfiram-like reaction with alcohol, and sun-sensitivity reactions are more common in first-generation sulfonylureas than in second-generation sulfonylureas.
Thiazolidinediones (Table 17-4)
Mechanism of action
Thiazolidinediones (also called glitazones or TZDs) are agonists of the PPARγ (peroxisome proliferators-activated receptor-γ) receptor, which, when stimulated, improves peripheral muscle and adipose tissue insulin sensitivity as well as suppresses hepatic glucose output.
Table 17-4. Thiazolidinediones

Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Clinical considerations
■ Minimal risk of hypoglycemia exists unless combined with secretagogues or insulin.
■ TZDs may cause a 5 kg weight gain—more if combined with secretagogues or insulin.
■ They decrease triglycerides (pioglitazone > rosiglitazone), increase HDL (pioglitazone = rosiglitazone), and increase LDL (rosiglitazone).
■ They are dosed daily, though rosiglitazone may be slightly more effective when dosed twice daily.
■ As much as 16 weeks of therapy may be required before assessing effectiveness.
■ Generally, they are not indicated during breastfeeding or pregnancy.
■ They may decrease the progression to DM from IGT and IFG.
■ Edema may best be treated by aldosterone antagonists.
■ They are not indicated by the FDA for treatment of type 2 DM in children, though they have been used.
■ They may be helpful in nonalcoholic fatty liver disease.
■ Conflicting data exist regarding risk of ischemic cardiovascular events caused by rosiglitazone.
A1C reduction
A reduction of 1–2% is expected.
Cautions and contraindications
■ Edema—with oral therapies (approximately 5%) and with insulin (approximately 15%)—may occur in patients with no history of heart problems. (The condition may be dose related.) Recommendation: Discontinue therapy if the problem is significant, decrease the dose if the problem is minor, and consider further cardiac workup.
■ They are contraindicated in patients with congestive heart failure.
■ Hepatotoxicity incidence is approximately 0.2% of alanine aminotransferase (ALT) > three times upper limit of normal (ULN) for both agents. Recommendation: Liver function tests (LFTs) every other month for first 12 months and periodically thereafter. If ALT > 2.5 ULN, do not start; if ALT = 1–2.5 ULN, monitor closely; if ALT = 3 × ULN, discontinue medication.
■ Pioglitazone has been linked to increased incidence of bladder cancer.
■ Medication may cause resumption of ovulation in anovulatory women.
■ Medication decreases oral contraceptive effectiveness.
■ The FDA proposed changes to rosiglitazone in the Risk Evaluation and Mitigation Strategy (REMS) in November 2013:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


