FIGURE 74-1 Interval plot showing multiple shocks for a patient with a lead fracture. The v-v intervals are less than 200 ms and are non-physiologic.
CASE EXPLANATION
• This is a case of nonphysiologic ventricular-sensed events on the ventricular lead resulting in the classification of ventricular fibrillation and delivery of therapy. Careful evaluation of all telemetered information is required to fully evaluate and classify therapy as appropriate or inappropriate.
• A complete evaluation includes assessment of lead integrity (sensing, impedance, and threshold values), lead parameter trends, device implant history, and imaging studies.
• The differential diagnosis of these events includes both physiologic and nonphysiologic events, such as ventricular fibrillation, lead noise, and electromagnetic interference (EMI).
ETIOLOGY/PATHOPHYSIOLOGY
The delivery of appropriate therapies is the pièce de résistance of ICDs. The delivery of multiple shocks can lead to great psychological distress; including depression for patients. When this occurs secondary to oversensing of nonphysiologic events, patients can develop fear and distrust of the reliability of the device designed to save their lives. Rapid intervention to prevent further shocks is essential. If therapy is found to be inappropriate secondary to noise, immediately inactivate all therapies while the patient and the device are evaluated.
The etiology of lead noise can be differentiated based on the number of leads demonstrating noise.
• Noise that is sensed on multiple leads suggests EMI.
• Noise sensed on a single lead can occur secondary to loose set screw, lead fracture, conduction failure, lead dislodgement, or air in the header.
Lead noise can represent lead integrity failure. In 2011, 150 386 new right ventricular ICD leads were implanted in the United States.1 Lead failure is estimated to occur in 0.58% per year of modern ICD lead implants.2
DIAGNOSIS
The approach to determining the etiology of noise artifact in this case includes assessing multiple aspects of the device.
• Clinical history: The patient’s history may provide key insight into the etiology of the noise artifact. Inquiry into possible sources of EMI should be made; including magnetic sources, TENS units, or operation of high voltage equipment.
• Implant information: Evaluate the device indication, implant techniques, leads used, or need for device revisions. Timing of noise from device implant or generator change should also be identified.
• Electrocardiogram (ECG): A 12-lead ECG should be performed on all patients with implantable devices. This allows for evaluation of the paced morphology. In particular, it is essential in patients with biventricular devices to assess pacing morphology. It is also useful to compare the implant ECG to the presenting ECG. Sudden change of a right ventricular-paced morphology from a left to right bundle branch block pattern may suggest lead migration or septal perforation.
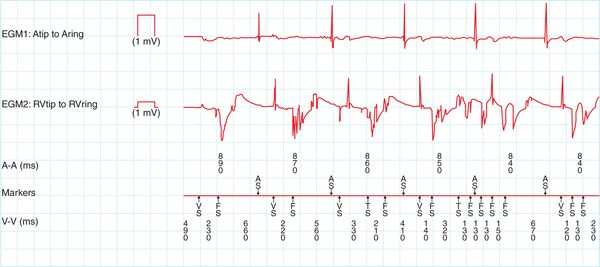
FIGURE 74-2 Intracardiac electrograms from patient with prior dot plot (74-1) showing noise. The tracing shows the typical sporadic, intermittent, high frequency non physiologic signals recorded from the sensed bipolar electrogram.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree