CHAPTER 59 Development of the thorax
THORACIC WALL AND DIAPHRAGM
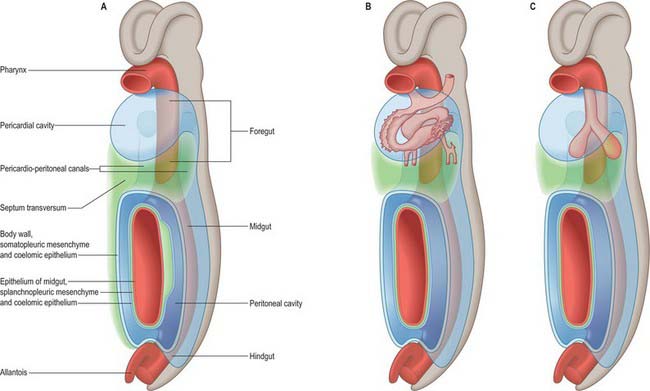
The thorax as an entity is not apparent in embryos until the end of the embryonic stage of development (stage 23). It develops around the early pericardial cavity and the associated pericardioperitoneal canals (Fig. 59.1A; see Fig. 59.3A). The pericardioperitoneal canals give rise to the pleural cavities surrounding the lungs and that part of the peritoneal cavity surrounding the lower end of the foregut, which becomes the oesophagus, stomach and duodenum within the thoracic cage. Later these portions of peritoneal cavity are sequestered into the abdominal cavity by the development of the diaphragm.
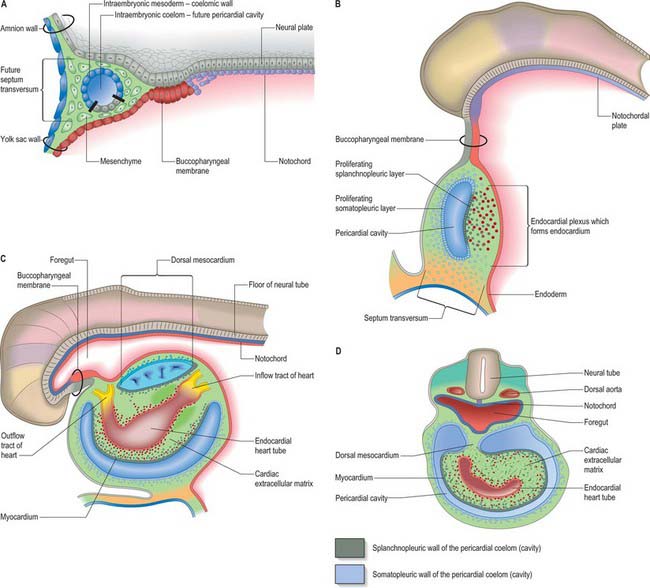
In stage 14 embryos, the heart is at the level of the upper cervical somites and above the upper limb buds. The thoracic somites are opposite the midgut. The putative thoracic region contains the pericardial cavity ventrally and the pericardioperitoneal canals posteriorly, on each side of the foregut. The future pleural cavities are as yet undefined regions of the pericardioperitoneal canals. Below the heart, septum transversum mesenchyme (see Fig. 73.5A) that has arisen from the caudal pericardial wall is being invaginated by endodermal epithelial cells from the foregut hepatic primordium.
The heart and pericardial cavity are relatively large in the early embryo (Fig. 59.1B; see Fig. 59.3A). Throughout development the lungs remain unexpanded and do not achieve their full size within the thorax, reflecting the fact that the placenta, and not the lungs, is the organ of fetal respiration. The lower partition of the thorax, the diaphragm, can be identified in stage 13 embryos; it migrates caudally in line with the craniocaudal progression of development and the elongation of the neck. The parietal pericardium remains attached to the diaphragm as it descends.
The lung buds are invested by splanchnopleuric mesenchyme derived from the medial walls of the pericardioperitoneal canals, whereas the lateral walls produce somatopleuric mesenchyme, which contributes to the body wall. This latter mesenchyme is penetrated by the developing ribs which arise from the thoracic sclerotomes. In the midline, the somatopleuric mesenchyme gives rise to the sternum and costal cartilages. The bony and cartilaginous cage provides insertions for the intercostal muscles, which arise from the ventrolateral edge of the epithelial plate of the somites. The somatopleuric coelomic epithelium, after its proliferative phase, gives rise to the mesothelium of the parietal layer of pleura.
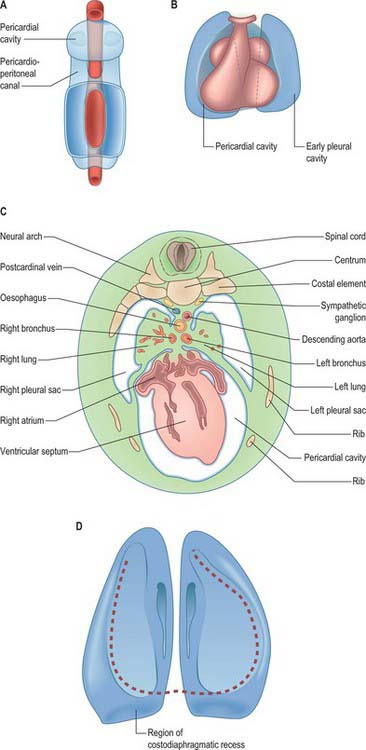
As the lung buds project into the pericardioperitoneal canals (Figs 59.1C, 59.2A), they subdivide them into primary pleural coeloms around the lung buds cranially, and paired peritoneal coeloms caudally, which are continuous with the wider peritoneal coelom around the mid- and hindguts. The communications with the pericardial and peritoneal coeloms become termed the pleuropericardial and pleuroperitoneal canals, respectively. When separation between these fluid-filled major coelomic regions is advancing towards completion, they are named the pericardial, pleural and peritoneal cavities. In early embryos, the cavities retain substantial volumes of fluid and their walls are separate: they provide the route for a primitive type of circulation until superseded by the blood vascular system. In later fetal and postnatal life, the cavity walls are coapted, so that a mere microscopic film of serous fluid intervenes between them.
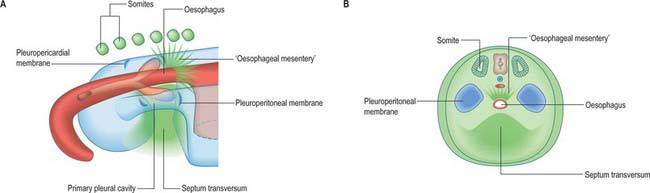
A curved elevation of tissue, the pulmonary ridge, develops on the lateral wall of the pleural coelom and partly encircles the pleuropericardial canal. The ridge is continuous with the dorsolateral edge of the septum transversum. The developing lung bud abuts on the ridge, which as a result divides into two diverging membranes meeting at the septum transversum. One is cranially placed and termed the pleuropericardial membrane (Fig. 59.2A); embedded within it are the common cardinal vein and phrenic nerve, which reach the septum transversum by this route. The other membrane, caudally placed, is termed the pleuroperitoneal membrane (Fig. 59.2A,B). As the apical part of the lung forms, it invades and splits the body wall and extends cranially on the lateral aspect of the common cardinal vein, preceded by an extension from the primary pleural coelom to form part of the secondary, definitive, pleural sac. In this way the common cardinal vein and the phrenic nerve come to lie medially in the mediastinum. The pleuropericardial canal, which lies medial to the vein, is gradually narrowed to a slit, which is soon obliterated by the apposition and fusion of its margins. Closure occurs early and is mainly effected by the growth and expansion of the surrounding viscera (heart and great vessels, lungs, trachea and oesophagus), and not by active growth of the pleuropericardial membrane across the opening to the root of the lung.
In addition to its extension in a cranial direction, the lung and its associated visceral and parietal pleurae also enlarge ventromedially and caudodorsally. With the ventromedial extension, the lungs and pleurae therefore excavate and split the somatopleuric mesenchyme over the pericardium, separating the latter from the ventral and lateral thoracic walls (Fig. 59.3B–D). The ventrolateral fibrous pericardium, parietal serous pericardium and mediastinal parietal pleura, although topographically deep, are therefore somatopleuric in origin.
Congenital disorders of the chest wall
The dorsal portion of the thorax is derived from somites, the sclerotomal portions of which form the thoracic vertebrae and ribs (Ch. 44). Vertebral anomalies, including formation of hemivertebrae and block vertebrae (where cranial and caudal sclerotomal halves do not separate), may contribute to scoliosis. Five percent of scoliosis is congenital; it may be associated with multiple other anomalies, e.g. the VACTERL (Vertebral; Anorectal; Cardiac; Tracheal; Esophageal; Renal; Limb) association or with congenital syndromes such as Marfan’s and congenital neurofibromatosis. The diagnosis may be missed at birth. There are a number of syndromes in which the chest wall does not develop properly, and the lungs are consequently hypoplastic. These include Jeune’s syndrome, asphyxiating thoracic dystrophy (an autosomal recessive condition which may be associated with short limb dwarfism and polydactyly). Rib cage abnormalities also occur in thanatrophic dwarfism, achondroplasia, chondroectodermal dysplasia and giant exomphalos. Many of these conditions are fatal soon after birth; milder forms may improve with time, but affected individuals may need prolonged respiratory support.
DIAPHRAGM
The separation of the pleural and peritoneal cavities is effected by development of the diaphragm, which forms from a portion of the septum transversum mesenchyme above the developing liver (Fig. 59.2A,B). The septum transversum is a population of mesenchymal cells which arises from the coelomic wall of the caudal part of the pericardial cavity. As the population proliferates, it forms a condensation of mesenchyme, caudal to the pericardial cavity and extending from the ventral and lateral regions of the body wall to the foregut. Dorsal to it on each side are the relatively narrow pleuroperitoneal canals. The endodermal hepatic bud grows into the caudal part of the septum transversum, whereas the cranial portion will form the diaphragm.
The oesophagus and stomach are medial to the pleuroperitoneal canals. As development proceeds, the lower portion of the oesophagus inclines ventrally anterior to the descending thoracic aorta. Although the oesophagus has no true ventral or dorsal mesentery, in descriptions of diaphragmatic development the portion of mesenchyme between the oesophagus and aorta at the level of the forming diaphragm is often homologized with part of a dorsal meso-oesophagus. The pleuroperitoneal membranes, which remain small, are dorsolateral to the canals, and the mesonephric ridges, suprarenal glands and gonads are dorsal. Just as the enlargement of the pleural cavity cranially and ventrally is effected by a process of burrowing into the body wall, so is its caudodorsal enlargement. The expanding pleural cavities extend into the mesenchyme dorsal to the suprarenal glands, the gonads and (degenerating) mesonephric ridges. Thus somatopleuric mesenchyme is peeled off the dorsal body wall to form a substantial portion of the dorsolumbar part of the diaphragm (Fig. 59.3C). The pleuroperitoneal canal is closed by the fusion of its edges, which are carried together from posterolaterally to anteromedially by growth of the organs surrounding it, in particular by growth of the suprarenal gland. The right pleuroperitoneal canal closes earlier than the left, which presumably explains why an abnormal communication persisting between the pleural and peritoneal cavities is more frequently encountered on the left.
While these changes are occurring, the septum transversum undergoes a progressive alteration in relative position. The dorsal border of the septum transversum which initially lies opposite the second cervical segment, migrates caudally as the embryo grows and the heart enlarges. At first the ventral border moves more rapidly than the dorsal, but after the embryo has attained a length of 5 mm, the dorsal border migrates more rapidly. When the dorsal border of the septum transversum lies opposite the fourth cervical segment, the phrenic nerve (C3, 4 and 5) and portions of the corresponding myotomes, grow into it and accompany it in its later migrations. The dorsal border of the septum transversum does not come to lie opposite the last thoracic and first lumbar segments, the final position occupied by some of the dorsal attachments of the diaphragm, until the end of the second month. The main derivatives of the central part of the diaphragm lie at considerably more cranial levels.
Diaphragmatic herniae
Diaphragmatic herniae may result from failure of fusion of the component parts or from a primary defect. Posterolateral defects (Bochdalek’s hernia) are the most common (85–90%) and may be bilateral (5%) or unilateral. Of the unilateral defects, the left side is more commonly affected (80%). Although these hernias have been attributed to failure of fusion of the pleuroperitoneal membrane, there is increasing evidence that the primary abnormality is lung hypoplasia, and the herniation of the abdominal contents is secondary, which has important implications for treatment (Jesudason 2002). A non-muscular membranous sac, possibly derived from the pleuroperitoneal canal wall is present in 10–15% of cases, signifying the early occurrence of this lesion prior to closure of the pleuroperitoneal canal. Hernias between the costal and sternal origins (Morgagni hernia) are rare (1–2%). Midline defects in the central tendon arise from septum transversum defects. The incidence of congenital diaphragmatic hernias is about 1 : 3000 to 1 : 5000 in neonates, with a prenatal incidence of 1 : 2000.
OESOPHAGUS
The development of the oesophagus is described in Chapters 35 and 73. Failure of separation of the oesophagus and trachea is described on page 1036. Oesophageal atresia and tracheo-oesophageal fistula may present antenatally with polyhydramnios due to failure of fetal swallowing, and choking and inability to swallow saliva in the neonatal period.
Neonatal thorax and diaphragm
In expiration, there may be active braking of airflow, caused by inspiratory muscle activity and partial constriction of the larynx: this produces grunting on expiration especially if the lungs are stiff, for example in neonatal respiratory distress. A very preterm neonate is difficult to study, but respiration is likely to be even more compromised by the compliant chest wall and lack of reserve than it is in the term newborn (Mortola 2002).
At all ages, there is a reduction, if not loss, of tonic intercostal activity during rapid eye movement (REM) sleep. The mechanism is believed to be related to a descending spinal inhibition of the muscle spindle system. In addition, although during REM sleep the diaphragm descends further, this inspiratory effort is dissipated in sucking in the ribs and enlarging the abdomen, thus the rib cage and abdominal ventilatory movements become out of phase. The neonate is at particular risk in this respect, because the chest wall is flexible, and much of the infant sleep activity is of the REM type. Furthermore, the upper airway musculature may lose tone during REM sleep, so that the soft tissues of the pharynx are sucked in during inspiration, limiting the cross-sectional area of the upper airway, and further increasing the work of breathing.
HEART AND GREAT VESSELS
CELLS THAT GIVE RISE TO THE HEART
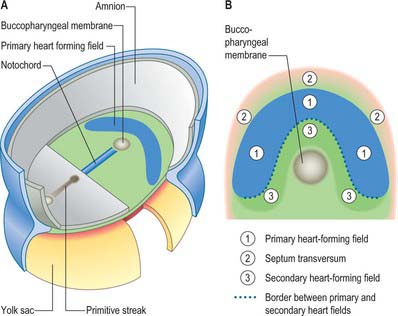
Primitive cardiac myocytes can first be seen in embryos at stage 9. During the onset of neurulation and somitogenesis, the intraembryonic coelom forms across the midline, initially above the endoderm, in a horse shoe-shaped area termed the cardiac crescent (Fig. 59.4). As the head fold emerges, the coelom undergoes a reversal, so that the future pericardial cavity comes to lie ventral to the endodermal foregut (Figs 59.1, 59.5). The splanchnopleuric wall of the pericardial coelom, subjacent to the endoderm, provides a germinal epithelium that produces early cardiac myocytes. It is characterized by the expression of myocardial specific markers, such as cardiac myosin heavy chain. This initial origin of myocardial cells is termed the primary heart-forming field, distinguishing it from later myocardial additions from mesenchyme localized central and peripheral to the cardiac crescent on the embryonic disc, termed the second heart-forming field (Fig. 59.4). It is not yet known whether these two sources of myocardium represent distinct myocardial lineages, or a single part which develops into separate components later on.
The endocardium also develops during stage 9 from the coelomic splanchnopleuric epithelium. Cells arise singly close to the ventro-lateral edges of the cranial intestinal portal and form an endocardial plexus between the splanchnopleuric coelomic epithelium and the foregut endoderm. These groups of cells are now termed angioblastic mesenchyme; they are amongst the earliest intraembryonic vascular precursors to appear and express markers for the endothelial cell lineage. The cells aggregate to form an epithelium, the endocardium, which encloses small cavities which coalesce in the vicinity of the developing foregut to establish bilateral, hollow endocardial tubes. The latter fuse across the midline progressively, commencing at the outflow tract, or arterial pole, and extending to the inflow tract, or venous pole (Fig. 59.5). By stage 10 a single endocardial tube is present and is almost completely surrounded by myocardial cells. This arrangement of an outer myocardial sleeve containing an inner endocardial tube constitutes the primary heart tube. The inner endocardial epithelium induces the myocardial cells to synthesize specific extracellular matrix proteins which form a fine extracellular reticulum that holds the endocardial tube apart from the developing myocardium. Close to the foregut endoderm, the myocardial cells at the reflections of the pericardial splanchnopleuric epithelium form the dorsal mesocardium, which may stabilize the developing endothelium and promote the fusion of the bilateral endocardial tubes. The dorsal mesocardium encompasses a mesenchymal population specifically referred to as mediastinal mesenchyme, and is contiguous with the splanchnopleuric mesenchyme surrounding the embryonic foregut.
The epicardium, sometimes included in descriptions of the myocardium as ‘epimyocardium’, is not present at the early stages of heart development (but see p. 1027).
Endocardial cushions
The extracellular matrix of the heart, historically termed cardiac jelly, promotes occlusion of the endocardial tubular lumen during myocardial contraction, thus providing mechanical assistance for the generation of the flow of blood. It also acts as a site for the deposition of inductive factors from the myocardial cells, which, in turn, modify the differentiation of specific endocardial cells. It has been called a gelatinoreticulum, a myoepicardial reticulum (Fig. 59.5C,D) and, more recently, the myocardial basement membrane. Here, the term cardiac extracellular matrix will be used. It is composed of, among other things, hyaluronic acid, hyaluronidase and fibronectin. Inductive signals originating from the myocardial cells cause a subset of endocardial cells lining the atrioventricular canal and the proximal outflow tract to transform into mesenchyme (cardiac mesenchyme); the endocardial cells in other regions of the heart tube, such as those in the ventricle, do not undergo such a transition. When activated by myocardial inductive factors, the endocardial cells lose their cell-to-cell associations, show decreased expression of neural cell adhesion molecule, and increased expression of substrate adhesion molecules such as chondroitin sulphate and fibronectin. They undergo rearrangement of their cytoskeleton necessary for migration, and they express type I procollagen. Uniquely, they retain their expression of endothelial markers. This epithelial to mesenchymal transition may, perhaps, be the only example of a mesenchymal population that is derived from an endothelial lineage.
Formation of cardiac mesenchymal cells at the atrioventricular canal and the proximal myocardial outflow tract is followed by their migration into the cardiac extracellular matrix. These cells proliferate between the endocardium and myocardium and, with local accumulation of extracellular matrix molecules, produce protrusions, termed endocardial or cardiac cushions, which bulge into the primary heart tube and initially provide the valvular mechanisms required in the atrioventricular canal and outflow tract. Their position corresponds to the future positions of the definitive cardiac valves. In the distal part of the outflow tract, which initially has myocardial walls, cells which are derived from the neural crest subsequently make significant contributions to the mesenchyme of the endocardial cushions. Although proper migration of these cells from the neural crest is crucial for normal development of the outflow tract and formation of the leaflets and sinuses of the arterial valves, their function is largely obscure. They are no longer found in the leaflets of the arterial valves in the formed heart, or in the muscular subpulmonary infundibulum, which is also derived from the outflow cushions. The endocardial cushions themselves ultimately fuse, forming a wedge of mesenchyme that serves to guide the union of the internal muscular septal structures (see p. 1024). At their time of fusion, the atrioventricular endocardial cushions are large relative to the size of the atrioventricular orifices; they will provide the scaffold for formation of the leaflets of the tricuspid and mitral valves.
Cardiac myocytes – contraction, conduction and automaticity
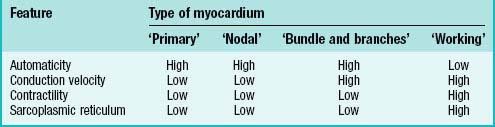
Cardiac myocytes share a number of characteristic features that distinguish them from other cells. All cardiac myocytes have sarcomeres and a sarcoplasmic reticulum and, in principle, share the capacity of producing an intrinsic cycle of electrical activity resulting in contraction. This phenomenon is called automaticity, or pacemaker activity. An absolute requirement for effective pacemaking is poor electrical coupling of the cells, which also implies slow conduction. It allows the cells to build up sufficient electrical charge which is then propagated through the surrounding myocardium. Thus ‘a small node can drive a large heart’ (see p. 1021).
Varying degrees of differentiation are seen in early populations of cardiac myocytes which can be categorized as forming working, nodal, conducting and primary myocardium (Table 59.1). Cells of the atrial and ventricular working myocardium display virtually no automaticity, but are well coupled and have well developed sarcomeres and sarcoplasmic reticular structures. The development of the synchronously (fast) contracting working myocardium requires fast conduction of the depolarizing impulse, and so the cells possess well-developed gap junctions. In marked contrast, the cells forming the nodes of the cardiac conduction system have the opposite phenotype, and resemble the myocytes which are found initially in the primary heart tube. The cells of the putative atrioventricular and peripheral ventricular conduction system have an ambiguous phenotype: the cells are well-coupled, thus allowing fast conduction of the depolarizing impulse, but otherwise retain an embryonic phenotype. The division does not imply that the cells belonging to one group are identical, but rather that they share distinguishing features developed to variable degrees.
HEART TUBE
During the process of embryonic folding, the midline splanchopleuric coelomic epithelium, which is derived from the primary heart field (cardiac crescent) becomes positioned ventral to the foregut. The splanchopleuric epithelium is proliferative and gives rise to mesenchymal populations and particularly early cardiac myocytes which arise from the coelomic wall adjacent to the endoderm of the foregut (Fig. 59.5). After folding, the second heart field contributes cells to both the arterial and venous poles of the heart. It has been suggested that the secondary heart field may contribute cells only to those cardiac components that are required for the pulmonary circulation, namely the right ventricle and outflow tract at the arterial pole, and the atrial septum and the dorsal atrial wall at the venous pole. Extending this suggestion, the original primary heart-forming field would give rise to those components that are required for the systemic circulation, namely the systemic venous sinus and its tributaries, the initial atrium, the left ventricle, and the arterial conus, as seen in the outflow tract of primitive fishes, although this latter structure has no homologue in mammalian hearts.
During the process of folding, the pericardial cavity and concomitantly the myocardium, gradually extend around the forming endocardial tube, leaving the dorsal mesocardium, a transient connection that is analogous to the mesentery of the intestines (Fig. 59.5C,D). The persisting stalk of the dorsal mesocardium connects the venous pole of the heart with the splanchnopleuric mesenchyme around the developing lung buds and with the septum transversum mesenchyme, which will give rise to the liver. The dorsal mesocardium is the site of early mediastinal mesenchyme production. It disappears as a mesenteric entity during the third week of development, when the embryo has from 4 to 12 somites; at the same time, the endocardial heart tube becomes entirely surrounded by the myocardium, and enclosed within the pericardial cavity. The breakdown of the dorsal mesocardium establishes a passage across the pericardial cavity, from side to side dorsal to the heart, which persists as the transverse sinus of the pericardium.
Looping of the heart tube
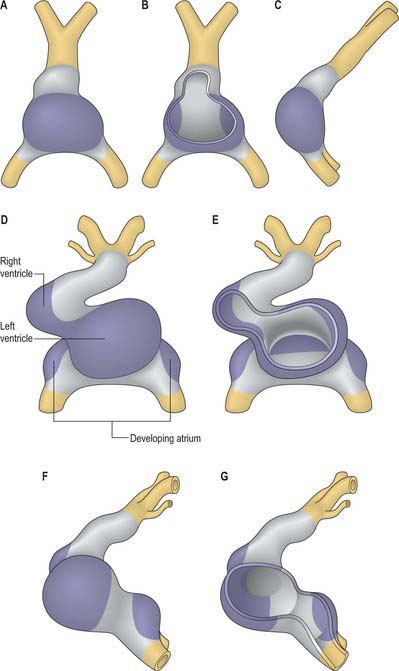
By the time the primary heart tube has formed its ventricular loop, it is possible to distinguish the atrial and ventricular components of the developing heart (Figs 59.6D-G, 59.7A,B) and to recognize an outflow tract connected with the aortic sac and developing pharyngeal arch arteries. The cells which, at this stage, make up the outflow tract, will, at later stages, be found within the right ventricle. At the proximal side of the outflow tract, cells are recruited to the developing right ventricle and, contemporaneously, new cells are recruited to the outflow tract from the second heart-forming field. The atrial and ventricular components are separated one from another by the atrioventricular canal, which at this stage has significant length. The systemic venous tributaries drain directly to the atrial cavity.
INFLOW TRACT
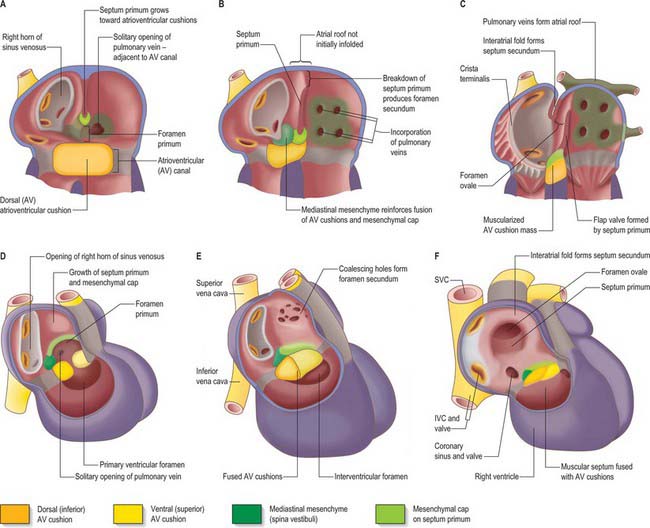
The working myocardium of the atrium differentiates locally at the dorsolateral sides of the heart tube (59.6D–G). The developing atrium then expands enormously, in dorsal, lateral and, most prominently, in a cranial direction. The cranial expansion is seen as pouches which become the left and right atrial appendages (Fig. 59.7A,B). The floor of the atrium, including the sinus venosus, and the atrioventricular canal, are made of primary myocardium. Less cellular proliferation occurs in these parts than in the expanding atrial chambers: the primary myocardium marks the inflow to, and the outflow from, the initial atrial chambers as ‘rings’ which are also associated with the formation of the conduction system (see p. 1021). The ring at the inflow defines the sinu-atrial junction, whilst the atrioventricular canal, which forms the atrial outlet during development, will eventually be incorporated into the definitive right and left atrial chambers as the atrial vestibules. Although it is possible to recognize the forming left and right atrial appendages at this stage, the right being more extensive than the left, the atrium itself has a single cavity and there is no evidence of septum formation. The myocardium of the sinus venosus and the newly forming mediastinal myocardium are smooth-walled, whereas the myocardium of the appendages shows ridges, the pectinate muscles, on the inner surface. The formation of the different appendages is under control of the Pitx2 signalling pathway. The myocardium of the appendages has a chamber phenotype, or is working myocardium; it expresses atrial natriuretic factor and connexin40 among other markers.
Right atrium
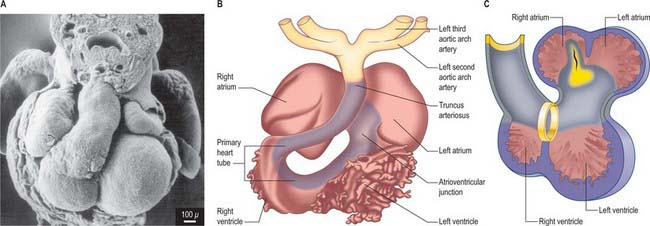
The further development of the right atrium is characterized by the incorporation of the sinus venosus into the right part of the primary atrium. This process is under control of the T-box transcription factor Tbx18. At about 4 weeks of development, the sinuatrial junction of the looping primary heart tube is positioned symmetrically in the midline (Fig. 59.8; see also Figs 13.1B, 73.8). The left and right common cardinal veins drain directly into the cavity of the primary atrium. The atrial myocardium extends to the margins of the pericardial cavity, and strictly speaking the sinus venosus is not yet formed, because the systemic venous tributaries are embedded within the mesenchyme of the septum transversum.
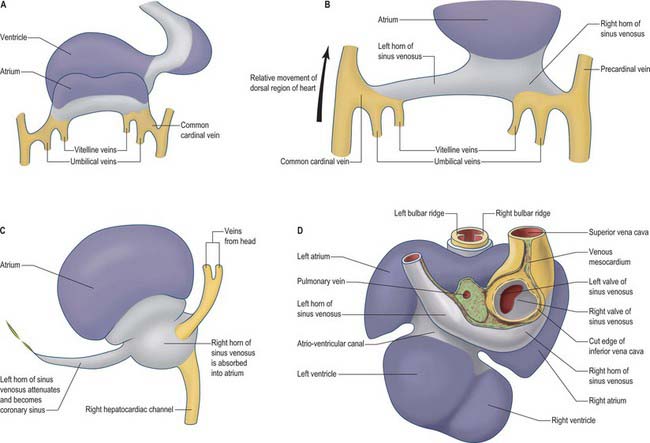
Fig. 59.8 The changes to the sinus venosus. A, Dorsal view of the early heart tube shown in Fig. 59.6D. B, Changes to the circulation brings the venous circulation to the right. This causes enlargement of the right horn of the sinus venosus and atrium and attenuation of the left horn of the sinus venosus. C, The right horn of the sinus venosus becomes absorbed into the atrium. The left horn of the sinus venosus becomes the coronary sinus. D, Dorsal view of the embryonic heart showing the relative changes to the sinus venosus.
During subsequent development, the pericardial cavity expands to enclose the terminal segments of the systemic venous tributaries, and at the same time their walls differentiate as myocardium. They can now be termed the left and right horns of the sinus venosus; each horn receives the union of the corresponding umbilical vein, vitelline radicles, and common cardinal vein (Fig. 59.8; see also Fig. 73.8). Concomitantly, the constriction between the left horn and the atrium becomes more pronounced. As the dorsal wall of the left atrium is formed from additions of mediastinal myocardium, the left horn becomes incorporated into the developing left atrioventricular junction, its orifice draining to the newly formed right atrium. At the same time, the left-sided venous tributaries diminish in size; the left common cardinal vein forms the oblique vein of the left atrium, and the left sinus horn forms the coronary sinus (Fig. 59.8), maintaining its own myocardial wall as it becomes incorporated into the atrioventricular junction.
The right sinus horn increases rapidly in size with growth of the liver (see Figs 73.8, 73.9). The vitello-umbilical blood flow enters the right horn through a wide but short hepatocardiac channel, which becomes the cranial end of the inferior vena cava. The right horn also receives the right common cardinal vein, draining the blood from the right side of the body (Fig. 59.8B,C). Later, when transverse connections are established between the cardinal veins, the blood from the left side of the body also reaches the heart via the veins draining the right side (see Fig. 13.4). As these changes take place, the right sinus horn, including the proximal parts of the superior and inferior cardinal veins, becomes incorporated into the right atrium, forming the smooth-walled systemic venous sinus, also known as the sinus venarum.
The right sinus horn opens into the right atrium through its dorsal and caudal walls (Fig. 59.8; see Fig. 59.12). The sinuatrial orifice becomes elongated and slit-like, guarded by two muscular folds, the left and right sinuatrial (venous) valves. These two valves meet cranially and become continuous with a fold that projects from the atrial roof, the septum spurium. The valves also meet caudally, and merge with the inferior atrioventricular cushion. With ongoing development, the cranial part of the right sinu-atrial valve loses its fold-like form, but its position is indicated in the adult heart by the site of the crista terminalis of the right atrium. Its caudal part forms the valve of the coronary sinus, also known as the Thebesian valve, and most of the valve of the inferior vena cava (Eustachian valve). The union of the two valvular remnants then passes through the tissue which separates the orifice of the coronary sinus from the fossa ovalis. This area is known as the ‘sinus septum’, but in reality this ‘septum’ is no more than a muscular fold in the dorsal wall of the right atrium. The continuation of the venous valves persists as the tendon of Todaro, an important landmark to the location of the atrioventricular node in the definitive heart. The left venous valve blends with the right side of the atrial septum; there is usually no trace of it in the postnatal heart.
Left atrium
Following the formation of the primary atrium and the left atrial appendage, the left atrium takes shape by the formation and incorporation of mediastinal myocardium. While the tributaries of the sinus venosus approach the atrium caudally, the differentiating pulmonary veins gain their entrance to the atrial cavity through the dorsal mesocardium (Fig. 59.5). The topographical relationships seen in the postnatal heart are thus established as soon as differentiation of the pulmonary venous portal occurs.
Early in the development of the atrium, the pulmonary vein develops as a solitary channel from angiogenic cells derived from the dorsal mesocardium, and establishes continuity with the vascular plexus formed in the mediastinal mesenchyme around the developing lung buds. The solitary pulmonary vein opens into the caudo-dorsal wall of the atrium adjacent to the developing atrioventricular junction, its atrial orifice being flanked by two prominent ridges. The primary atrial septum develops from the right ridge, following incorporation of the left sinus horn into the right side of the primary atrium, thus confining the pulmonary venous orifice to the developing left atrium (Fig. 59.12). The pulmonary vein initially branches within the dorsal mediastinal mesenchyme, its tributaries draining blood from the developing lung. With continuing development, the walls of the venous channels become surrounded by myocardium, this process occurring to the level of the second bifurcation. The veins then expand, and are incorporated into the roof of the left atrium, eventually forming the greater part of its cavity. However, all four pulmonary veins do not achieve their separate opening into the atrial roof until well after the completion of atrial septation, and probably not until the 10th or 11th week of development. Variations in the precise pattern of pulmonary venous drainage are quite common. With these changes, the left half of the primary atrium becomes progressively restricted to the mature appendage. The myocardial sleeves surrounding the pulmonary orifices taper off, and become intermingled with fibrous tissue. In later life, it is likely that this intermingling of myocardial and fibrous tissues forms the substrate for some forms of atrial fibrillation. The opening of the solitary pulmonary vein to the left of the right pulmonary ridge is an essential pre-requisite for atrial septation.
VENTRICLES
The ventricles develop at the ventral side of the looping primary heart tube during the fourth week of development (Figs 59.6, 59.7). The left ventricle develops from the stem of the Y-shaped heart, the right ventricle develops later, downstream relative to the left ventricle, when more myocardium has been added to the cardiac tube. As a result of the looping of the heart tube, the right ventricle is positioned at the right of the left ventricle, which is a prerequisite for the appropriate connection with the expanding atrial component of the heart. Unlike the atrial chambers, the morphological differences between the right and left ventricles are not part of the general asymmetry between the right- and left-sided organs of the body, but rather are under control of the signalling pathways which determine caudo-cranial differentiation. Retinoic acid, and its downstream transcription factor Tbx5, play a crucial role in this process.
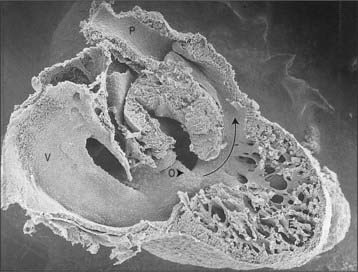
The myocardium at the inner curvature, the original dorsal side of the cardiac tube, remains smooth-walled and maintains its molecular phenotype, whereas the myocardium at the outer curvature of the myocardial tube displays trabeculations in the fifth week of development (Fig. 59.7B). By stage 17, the trabeculations have achieved a typical spatial orientation, giving a sponge-like appearance to the internal aspect of both ventricles (Fig. 59.9). The definitive trabeculations, coarse in the right ventricle but much finer in the left, are first observed about the 40th day of gestation: they appear initially in the walls of both ventricles at the level of the atrioventricular junction and develop towards the apex of the heart. By the time the fetus is 10 weeks old, the trabeculations are much sparser, and are confined to the apical regions. This process of remodeling is accomplished without the intervention of macrophages or inflammatory cells in the immediate interstitium. The ventricular myocardium, encompassing the trabeculations and exterior wall, possesses a chamber phenotype, the myocytes expressing, among other proteins, the gap-junctional protein Cx40 and atrial natriuretic peptide. This myocardium stops proliferating and differentiates into the fast-conducting peripheral ventricular conduction system, whereas the outer layer becomes highly proliferative and forms the compact layer of the ventricular wall.