48
Dermatological Pharmacology
This chapter will be most useful after having a basic understanding of the material in Chapter 65, Dermatological Pharmacology in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• A detailed description of the structures of the skin and their pharmacological implications
• A discussion of antimicrobial therapy of skin disorders
• A discussion of the use of cytotoxic and immunosuppressive drugs in the treatment of skin disorders
• Tables 65-4 and 65-5 which are a listing of topical and systemic retinoids, respectively
• Chemical structures of drugs used to treat dermatological disorders
LEARNING OBJECTIVES
 Understand how drugs are absorbed through the skin.
Understand how drugs are absorbed through the skin.
 Know the mechanisms of action, therapeutic uses, and toxicities of topical and systemic drugs used to treat dermatological disorders.
Know the mechanisms of action, therapeutic uses, and toxicities of topical and systemic drugs used to treat dermatological disorders.
 Know the principles of photochemotherapy of dermatological disorders.
Know the principles of photochemotherapy of dermatological disorders.
 Know the science behind the use of sunscreen agents.
Know the science behind the use of sunscreen agents.
DRUGS INCLUDED IN THIS CHAPTERa
• Acitretin (SORIATANE)
• Adalimumab (HUMIRA)
• Adapalene (DIFFERIN)
• Alefacept (AMEVIVE)
• Alitretinoin (9-cis-retinoic acid) (PANRETIN)
• Aminolevulinic acid (ALA, LEVULAN KERASTICK)
• Azathioprine (IMURAN, others)
• Azelaic acid (AZELEX, FINACEA)
• Benzyl alcohol
• Bexarotene (TARGRETIN)
• Bleomycin (BLENOXANE)
• Calcipotriene (DOVONEX, others)
• Capsaicin (ZOSTRIX, CAPSIN)
• Carmustine (BICNU)
• Chloroquine (ARALEN, others)
• Crotamiton (EURAX)
• Cyclophosphamide
• Cyclosporine (NEORAL, GENGRAF, others)
• Dapsone (diaminodiphenylsulfone, DDS)
• Denileukin Diftitox (ONTAK)
• Doxorubicin (DOXIL, CAELYX)
• Efalizumab (RAPTIVA)
• Etanercept (ENBREL)
• Finasteride (PROPECIA, others)
• Fluorouracil (CARAC, others)
• Hydroquinone (TRI-LUMA)
• Hydroxychloroquine (PLAQUENIL, others)
• Imiquimod (ALDARA)
• Infliximab (REMICADE)
• Isotretinoin (13-cis-retinoic acid) (ACCUTANE)
• Ivermectin (STOMECTOL)
• Lindane
• Malathion (OVIDE)
• Mechlorethamine (MUSTARGEN)
• Mequinol (SOLAGE, in combination with tretinoin and vitamin C)
• Methotrexate (RHEUMATRIX)
• Methoxsalen (OXSORALEN, others)—not marketed in the United States
• Methylaminolevulinate (MAL, VETVIXIA)
• Minoxidil (ROGAINE, others)
• Monobenzone (BENOQUIN)
• Mycophenolate mofetil (CELLCEPT)
• Permethrin (KLOUT shampoo)
• Pimecrolimus (ELIDEL)
• Podophyllin (podophyllum resin)
• Quinacrine (ATABRINE)
• Retapamulin (ALTABAX)
• Retinol (vitamin A)
• Tacrolimus (FK506, PROTOPIC)
• Tazarotene (TAZORAC, others)
• Thalidomide (TALOMID)
• Tretinoin (all trans-retinoic acid; vitamin A acid) (ATRALIN, others)
• Triamcinolone acetonide (KENALOG-10)
• Triamcinolone hexacetonide (ARISTOSPAN)
• Vinblastine (VELBAN)
aDrugs included in this chapter have specific dermatological uses. Antibiotics used to treat acne are listed in the Side Bar ANTIBIOTICS USED TO TREAT ACNE. Antimicrobials used to treat cutaneous infections are discussed in the cases; the pharmacology of specific drugs can be found in Chapters 35, 39, 40, 41, 43, and 44. Sunscreen agents are listed in the Table SUNSCREEN AGENTS.
MECHANISMS OF ACTION
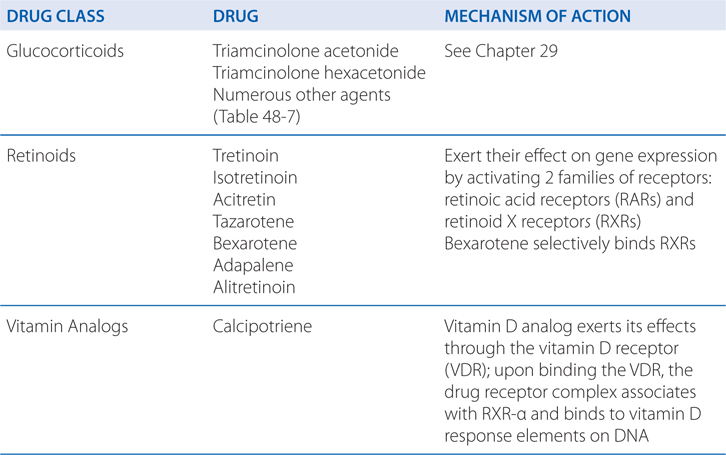
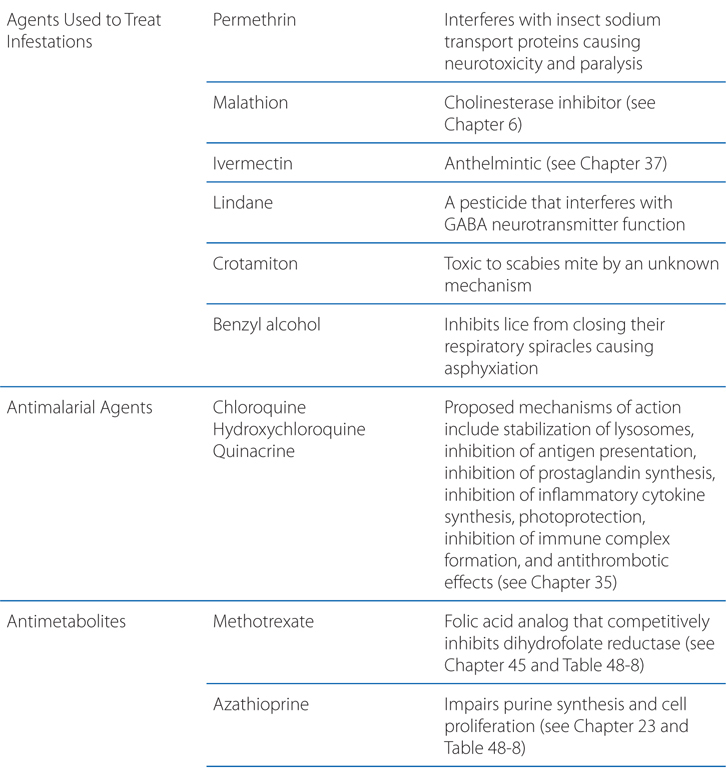
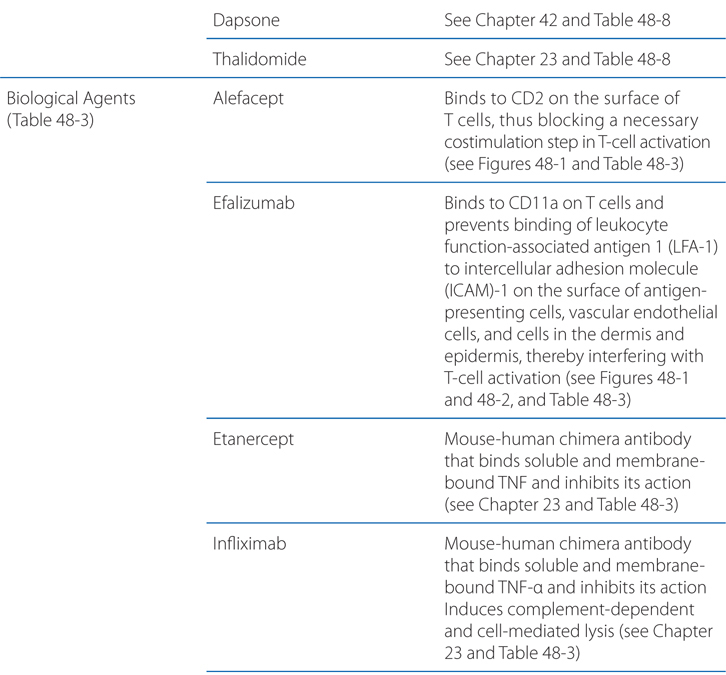
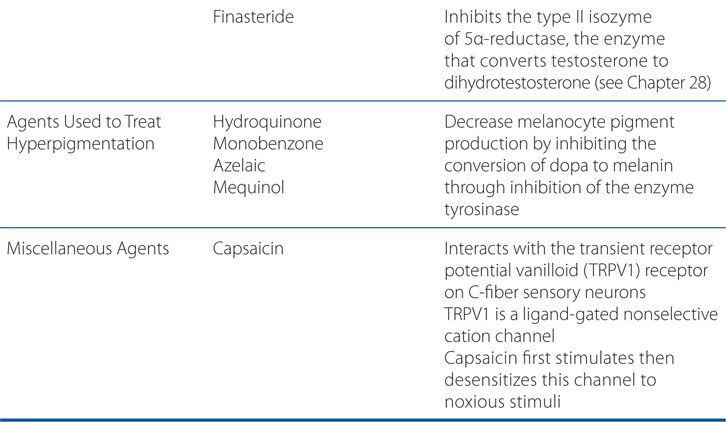
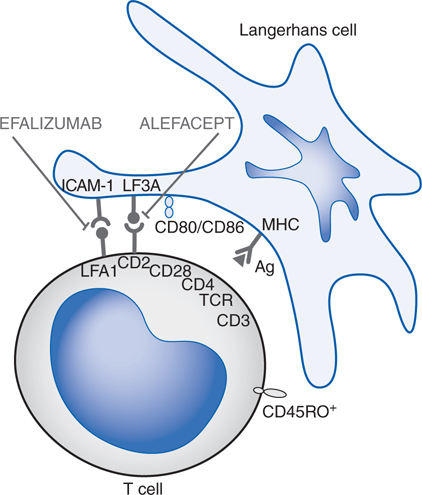
FIGURE 48-1 Mechanisms of action of selected biological agents in psoriasis. Newer biological agents can interfere with one or more steps in the pathogenesis of psoriasis, resulting in clinical improvement. See text in Chapter 65, Dermatological Pharmacology in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition for details. ICAM-1, intercellular adhesion molecule 1; LFA, lymphocyte function–associated antigen; MHC, major histocompatibility complex; TCR, T-cell receptor.
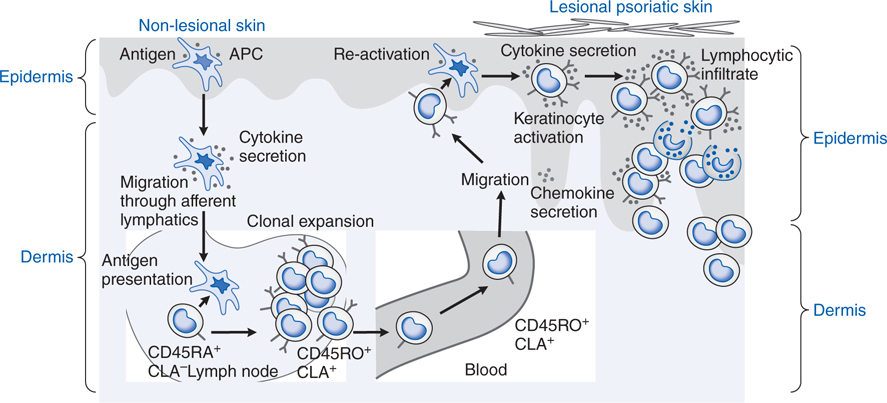
FIGURE 48-2 Immunopathogenesis of psoriasis. Psoriasis is a prototypical inflammatory skin disorder in which specific T-cell populations are stimulated by as-yet undefined antigen(s) presented by antigen-presenting cells. The T cells release proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), that induce keratinocyte and endothelial cell proliferation. APC, antigen-presenting cell; CLA, cutaneous lymphocyte-associated antigen.
MECHANISMS OF PERCUTANEOUS ABSORPTION
The process of absorption of a topically applied drug consists of:
• Establishment of a concentration gradient
• Movement of drug into stratum corneum (partition coefficient)
• Diffusion of drug across layers of skin (diffusion coefficient)
The relationship of these factors is summarized in the following equation:
J ∝ Cveh ⋅ Km ⋅ D/X
where J is the rate of absorption; Cveh is the concentration of drug in the vehicle; Km is the partition coefficient; D is the diffusion coefficient; and x is the thickness of the stratum corneum
A 70-year-old woman has developed an infection from scratching an area on her leg. Despite her physician’s recommendation for an oral antibiotic, she wishes to have a “cream” to put on it.
a. What is the process of absorption of the antibiotic through the skin?
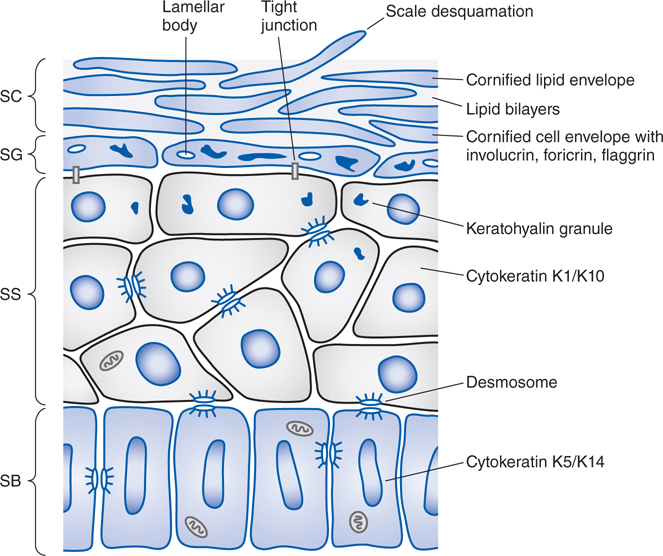
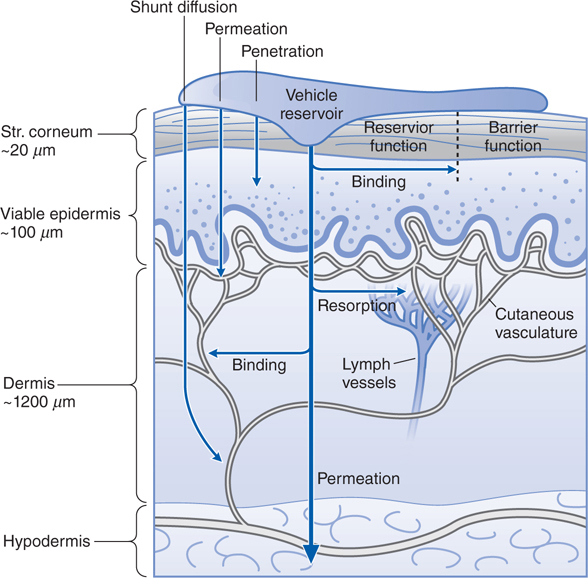
Passage through the outermost layer is the rate-limiting step for percutaneous absorption. Figure 48-3 shows the structure and layers of the skin, and Figure 48-4 shows the compartments of skin as they relate to cutaneous drug delivery. The mechanisms of percutaneous absorption are shown in the Side Bar MECHANISMS OF PERCUTANEOUS ABSORPTION.
FIGURE 48-3 Structure of the epidermis. The epidermis matures progressively from the stratum basale (SB) to the stratum spinosum (SS), stratum granulosum (SG), and stratum corneum (SC). Important structural and metabolic proteins are produced at specific layers of the epidermis. (Reproduced with permission from Wolff K et al (eds). Fitzpatrick’s Dermatology in General Medicine, 7th ed. McGraw-Hill, Inc., 2008. Figure 45-2.)
FIGURE 48-4 Cutaneous drug delivery. Diagrammatic representation of the 3 compartments of the skin as they relate to drug delivery: surface, stratum (Str.), and viable tissues. After application of drugs to the surface, evaporation, structural, and compositional alterations, which determine the bioavailability of drugs, occur in the applied formulation. The stratum corneum limits diffusion of compounds into the viable skin and body. After absorption, compounds either bind targets in viable tissues or diffuse within the viable tissue or into the cutaneous vasculature, where they may be carried to internal cells and organs. (Reproduced with permission from Wolff K et al (eds). Fitzpatrick’s Dermatology in General Medicine, 7th ed. McGraw-Hill, Inc., 2008. Figure 215-1.)
b. What are the important considerations when applying a drug to the skin?
The important considerations when applying a drug to the skin are shown in Table 48-1. In addition, the following considerations should be taken into account: dosage, under-application of a drug because of cost considerations often occurs when large amounts of skin are treated for a long time; regional anatomical variation, drug penetration is higher on the face, intertriginous areas, and perineum due to stratum corneum thickness (see Figures 48-3 and 48-4). Skin sites that are naturally occluded by apposing surfaces, such as the axillae, groin, and inframammary areas, are vulnerable to drug-related atrophy from potent topical glucocorticoids; altered barrier function in disease, in many dermatological diseases, the stratum corneum is abnormal and barrier function is compromised. In these settings, increased percutaneous absorption of potent topical steroids can cause systemic toxicity, such as hypothalamic–pituitary–adrenal (HPA) axis suppression; vehicle, drug vehicles are summarized in Table 48-2 and below in the answer to Case 1c; age, children have a greater ratio of surface area to mass than adults do, so the same amount of topical drug can result in a greater systemic exposure; application frequency, topical agents often are applied twice daily. For certain drugs, once-daily application of a larger dose may be equally effective as more frequent applications of smaller doses. For some drugs, the stratum corneum may act as a drug reservoir that allows gradual penetration into the viable skin layers over a prolonged period; intralesional administration, intralesional drug administration is used mainly for inflammatory lesions but also can be used for treatment of warts and selected neoplasms. Medications injected intralesionally have the advantages of direct contact with the underlying pathological process, no first-pass metabolism, and the opportunity for a slowly absorbed depot of drug.
TABLE 48-1 Important Considerations When a Drug Is Applied to the Skin
What are the absorption pathways of intact and diseased skin?
How does the chemistry of the drug affect the penetration?
How does the vehicle affect the penetration?
How much of the drug penetrates the skin?
What are the intended pharmacological targets?
What host and genetic factors influence drug function in the skin?
TABLE 48-2 Vehicles for Topically Applied Drugs
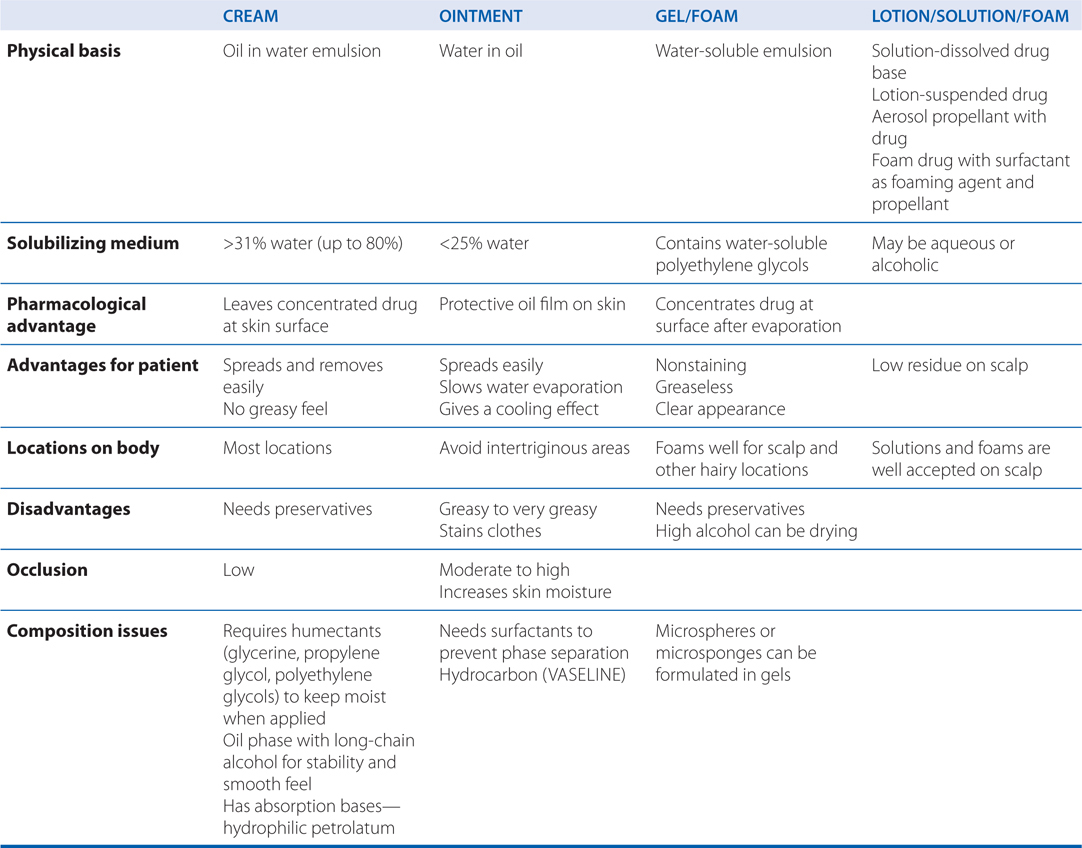
c. What vehicles are available for topically applied drugs?
Vehicles for the topical administration of drugs are shown in Table 48-2. Newer vehicles include liposomes and microgel formulations. Liposomes are concentric spherical shells of phospholipids in an aqueous medium intended to enhance percutaneous absorption in normal and abnormal stratum corneum. Variations in size, charge, and lipid content can influence liposome function substantially. Transfersomes are a drug-delivery technology based on highly deformable, ultra-flexible lipid vesicles that penetrate the skin when applied nonocclusively. Microgels are polymers intended to enhance solubilization of certain drugs, thereby enhancing topical penetration and diminishing irritancy.
d. What concerns should the physician have when treating an infection of the skin with topical therapy?
Gram-positive organisms, including Staphylococcus aureus and Streptococcus pyogenes, are the most common cause of pyoderma. Skin infections with gram-negative bacilli are rare, although they can occur in diabetics and patients who are immunosuppressed; appropriate parenteral antibiotic therapy is required for their treatment.
Topical therapy frequently is adequate for impetigo, a superficial bacterial infection of the skin caused by S. aureus and S. pyogenes. Topical therapy often is employed for prophylaxis of superficial infections caused by wounds and injuries.
Deeper bacterial infections of the skin include folliculitis, erysipelas, cellulitis, and necrotizing fasciitis. Because streptococcal and staphylococcal species also are the most common causes of deep cutaneous infections, penicillins (especially β-lactamase–resistant β-lactams) and cephalosporins are the systemic antibiotics used most frequently in their treatment (see Chapter 39). A growing concern is the increased incidence of skin and soft-tissue infections with hospital- and community-acquired methicillin-resistant S. aureus (MRSA) and drug-resistant pneumococci. Infection with community-acquired MRSA often is susceptible to trimethoprim–sulfamethoxazole. See previous chapters for the pharmacology of specific antibacterial agents.
A 35-year-old man has the diagnosis of psoriasis. He is being treated with methotrexate.
a. What is psoriasis?
Psoriasis is a disorder of Th1 cell-mediated immunity (see Figure 48-2), with the epidermal changes being secondary to the effect of released cytokines.
b. Why is methotrexate efficacious in the treatment of psoriasis?
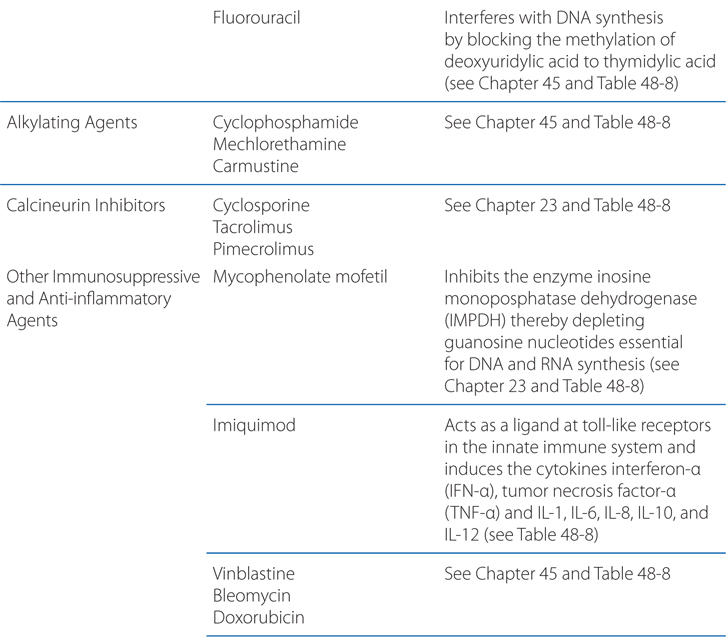
The antimetabolite methotrexate is a folic acid analog that competitively inhibits dihydrofolate reductase (see Chapter 45). Methotrexate has been used for moderate to severe psoriasis since 1951. It suppresses immunocompetent cells in the skin, and it also decreases the expression of cutaneous lymphocyte-associated antigen (CLA)–positive T cells and endothelial cell E-selectin, which may account for its efficacy.
c. What adverse effects of methotrexate might limit its use?
Doses of methotrexate must be decreased for patients with impaired renal clearance. Methotrexate should never be co-administered with trimethoprim–sulfamethoxazole, probenecid, salicylates, or other drugs that can compete with it for protein binding and thereby raise plasma concentrations to levels that may result in bone marrow suppression. Fatalities have occurred because of concurrent treatment with methotrexate and nonsteroidal anti-inflammatory agents. Methotrexate exerts significant antiproliferative effects on the bone marrow; therefore, a complete blood count should be monitored serially. Physicians administering methotrexate should be familiar with the use of folinic acid (leucovorin) to rescue patients with hematological crises caused by methotrexate-induced bone marrow suppression. Careful monitoring of liver function tests is necessary but may not be adequate to identify early hepatic fibrosis in patients receiving chronic methotrexate therapy. Methotrexate-induced hepatic fibrosis may occur more commonly in patients with psoriasis than in those with rheumatoid arthritis. Consequently, liver biopsy is recommended when the cumulative dose reaches 1 to 1.5 g. A baseline liver biopsy also is recommended for patients with increased potential risk for hepatic fibrosis, such as a history of alcohol abuse or infection with hepatitis B or C. Patients with significantly abnormal liver function tests, symptomatic liver disease, or evidence of hepatic fibrosis should not use this drug. Many clinicians routinely administer folic acid along with methotrexate to ameliorate side effects; this does not reduce efficacy of the methotrexate. Pregnancy and lactation are absolute contraindications to methotrexate use.
d. What other therapeutic options are available for this patient?
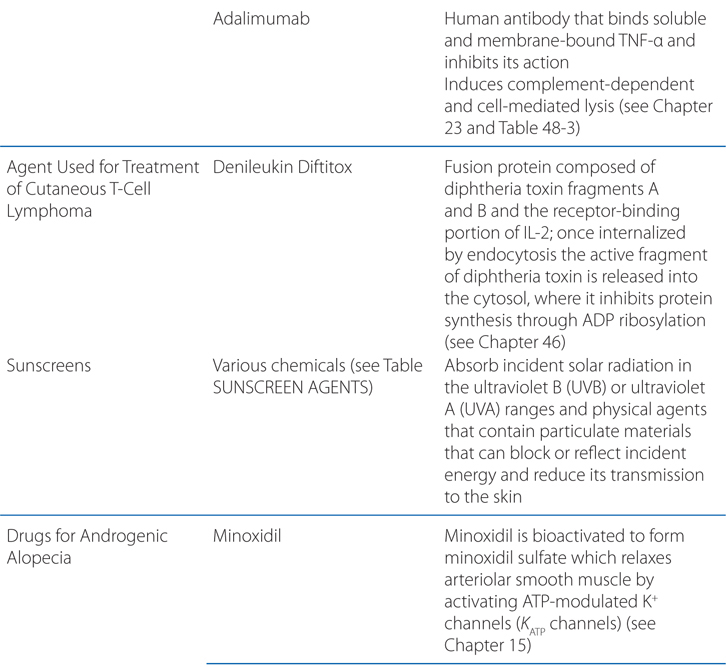
Biological agents (see Chapter 23) are compounds derived from living organisms that target specific mediators of immunological reactions. Classes of biologicals include recombinant cytokines, interleukins, growth factors, antibodies, and fusion proteins. Currently, 5 biological agents are approved for the treatment of psoriasis (see Table 48-3). Biological therapies modify the immune response in psoriasis through (1) reduction of pathogenic T cells, (2) inhibition of T-cell activation, (3) immune deviation (from a Th1 to a Th2 immune response), and (4) blockade of the activity of inflammatory cytokines.
TABLE 48-3 Biological Agents Commonly Used in Dermatology
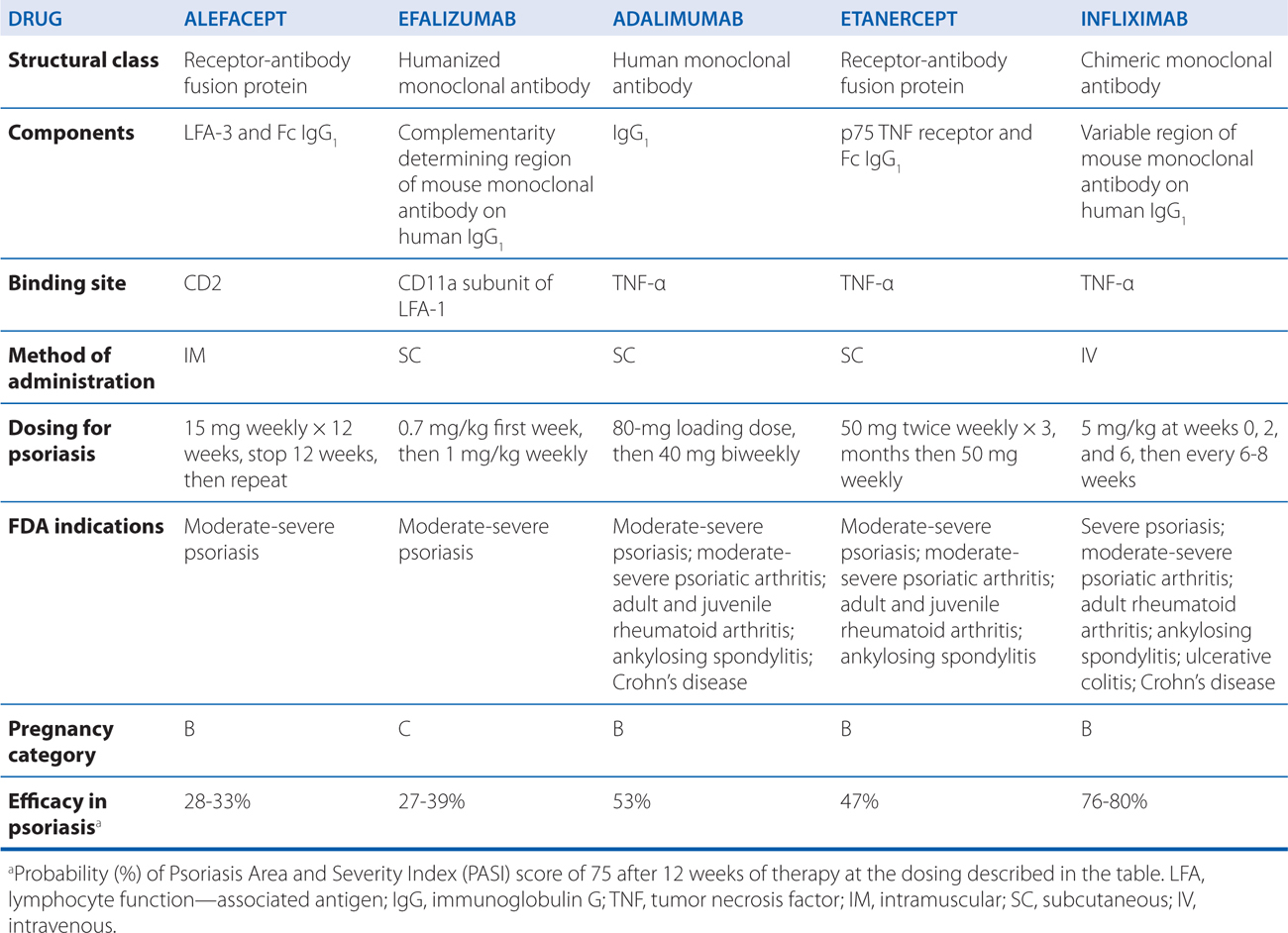
The appeal of biological agents in the treatment of psoriasis is that they specifically target the activities of T lymphocytes and cytokines that mediate inflammation versus traditional systemic therapies that are broadly immunosuppressive or cytotoxic. Thus, the use of these agents theoretically should result in fewer toxicities and side effects.
When evaluating the efficacy of biological agents, it is important to understand the standard measurement of efficacy in psoriasis treatment, the Psoriasis Area and Severity Index (PASI). The PASI quantifies the extent and severity of skin involvement in different body regions as a score from 0 (no lesions) to 72 (severe disease). To gain FDA approval for the treatment of psoriasis, a biological agent must decrease the PASI by 75%. Although such quantification is an essential element in controlled clinical trials, many patients in practice may gain clinically significant benefit from biological treatment without achieving this degree of PASI improvement.
Prior to his treatment with methotrexate, the patient in Case 48-2 was treated with photochemotherapy.
a. What is photochemotherapy?
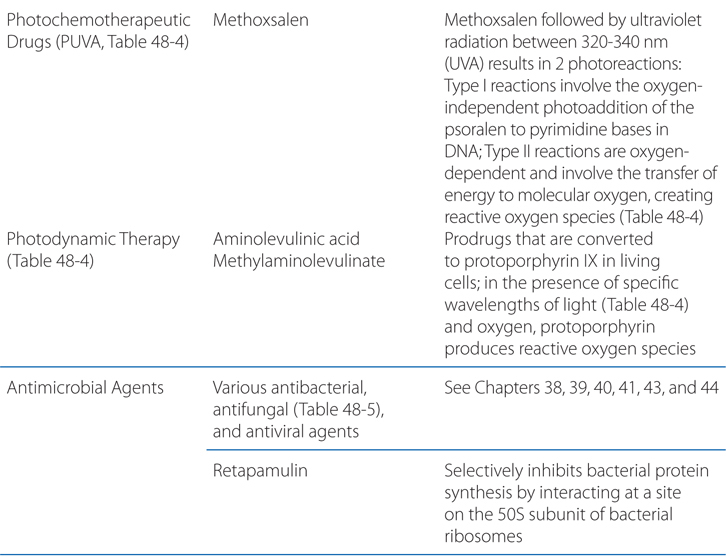
Phototherapy and photochemotherapy are treatment methods in which UV or visible radiation is used to induce a therapeutic response either alone or in the presence of a photosensitizing drug. To be effective, the incident radiation must be absorbed by a target or chromophore in the skin—which in phototherapy is endogenous and in photochemotherapy must be administered exogenously (see Table 48-4).
TABLE 48-4 Photochemotherapy Methods
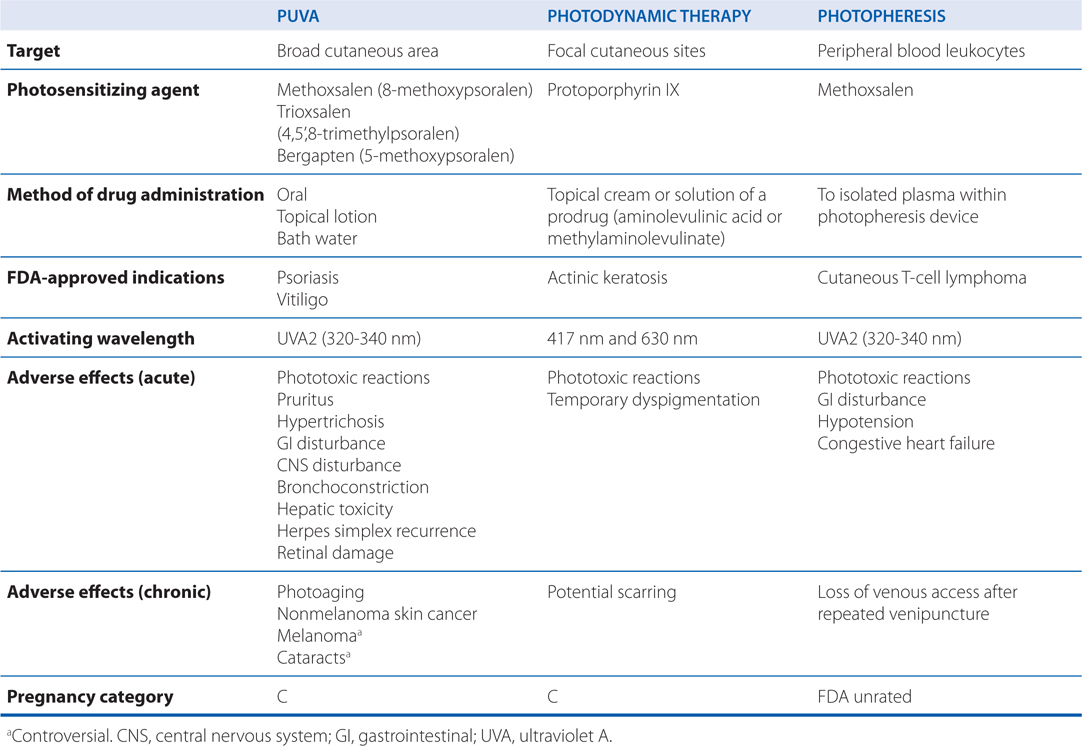
The action spectrum for oral psoralen (eg, methoxysalen) followed by ultraviolet light A (PUVA) is between 320 and 340 nm. Two distinct photoreactions take place. Type I reactions involve the oxygen-independent photoaddition of psoralens to pyrimidine bases in DNA. Type II reactions are oxygen-dependent and involve the transfer of energy to molecular oxygen, creating reactive oxygen species. Through incompletely understood mechanisms, these phototoxic reactions stimulate melanocytes and induce antiproliferative, immunosuppressive, and anti-inflammatory effects.
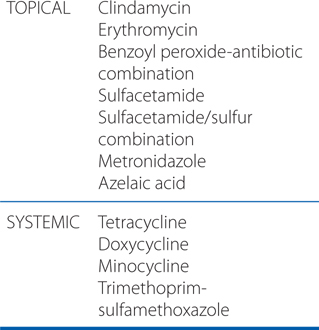
ANTIBIOTICS USED TO TREAT ACNE
b. What are the potential toxicities of phototherapy and photochemotherapy and how should patients be monitored?
Patients treated with these modalities should be monitored for concomitant use of other potential photosensitizing medications before initiation of therapy. Such drugs include phenothiazines, thiazides, sulfonamides, nonsteroidal anti-inflammatory agents, sulfonylureas, tetracyclines, and benzodiazepines.
The major side effects of PUVA are listed in Table 48-4. Phototoxicity is characterized by erythema, edema, blistering, and pruritus. Ocular toxicity can be prevented by wearing UVA-blocking glasses the day of treatment. The risk of nonmelanoma skin cancer is dose-dependent, with the greatest risk in those receiving more than 250 treatments. A possible association of melanoma and extensive exposure to PUVA has been reported; however, several other studies have failed to confirm this association. As skin cancer may not develop for decades after exposure, annual skin examinations should be continued for years after completion of PUVA.
A 17-year-old boy with noninflammatory acne is treated with a topical retinoid preparation.
a. What is acne?
Acne is believed to result from a combination of sebaceous gland hyperplasia, follicular hyperkeratosis, Propionibacterium acnes colonization, and inflammation. Though incompletely understood mechanisms, topical retinoids correct abnormal follicular keratinization, reduce P. acnes counts, and reduce inflammation, thereby making them the cornerstone of acne therapy. Topical retinoids are first-line agents for noninflammatory (comedonal) acne and often are combined with other agents in the management of inflammatory acne.
b. What is the mechanism of action of retinoids?
Retinoids exert their effects on gene expression by activating 2 families of receptors—retinoic acid receptors (RARs) and retinoid X receptors (RXRs)—that are members of the steroid receptor superfamily.
Unique therapeutic effects can be produced by targeting specific retinoid receptors. For example, retinoids that target RARs predominantly affect cellular differentiation and proliferation, whereas retinoids that target RXRs predominantly induce apoptosis. Hence, tretinoin, adapalene, and tazarotene, which target RARs, are used in acne, psoriasis, and photoaging (disorders of differentiation and proliferation), whereas bexarotene and alitretinoin, which target RXRs, are used in mycosis fungoides and Kaposi sarcoma (to induce apoptosis of malignant cells).
c. What retinoid preparations are available for this patient?
Topical and systemic retinoids are shown in Tables 65-4 and 65-5, respectively, in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition.
d. What adverse effects might this patient experience from retinoids?
Acute retinoid toxicity is similar to vitamin A intoxication. Side effects of systemic retinoids include dry skin, nosebleeds from dry mucous membranes, conjunctivitis, reduced night vision, hair loss, alterations in serum lipids and transaminases, hypothyroidism, inflammatory bowel disease flare, musculoskeletal pain, pseudotumor cerebri, and mood alterations. RAR-selective retinoids are more associated with mucocutaneous and musculoskeletal symptoms, whereas RXR-selective retinoids induce more physiochemical changes. Suicide or suicide attempts have been associated with the use of isotretinoin. Thus, all patients treated with isotretinoin should be observed closely for symptoms of depression or suicidal thoughts.
Adverse effects of all topical retinoids include erythema, desquamation, burning, and stinging. These effects often decrease with time and are lessened by concomitant use of emollients. Patients also may experience photosensitivity reactions because of enhanced reactivity to UV radiation and have a significant risk for severe sunburn. See Summary Quiz Question 48-3 for a discussion of the avoidance of retinoids during pregnancy.
e. What other drugs are available for the treatment of acne?
Antibiotics are commonly used to treat acne. Commonly used antibiotics are shown in the Side Bar ANTIBIOTICS USED TO TREAT ACNE.
A 53-year-old woman has widespread tinea corporis infection in her skin that is causing irritation and itching. Her physician has prescribed oral fluconazole.
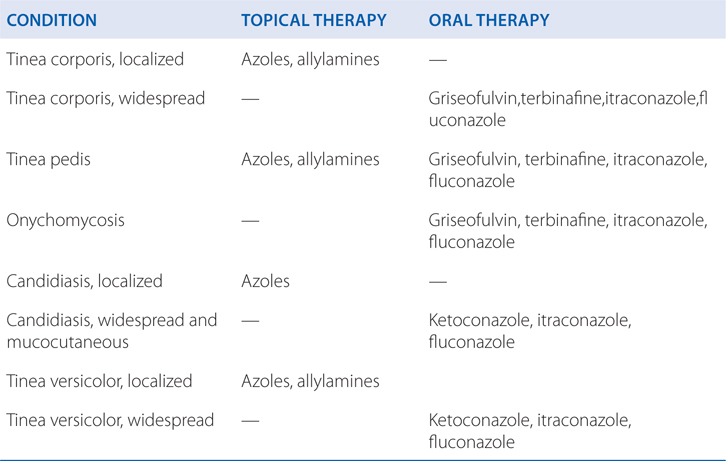
a. What preparations are available for the treatment of cutaneous fungal infections?
Table 48-5 shows the recommendations for cutaneous antifungal therapy.
TABLE 48-5 Recommended Cutaneous Antifungal Therapy
b. What are the adverse effects of fluconazole?
The azoles interact with hepatic CYPs as substrates and inhibitors, providing myriad possibilities for the interaction of azoles with many other medications. See Chapter 43 for a complete discussion of the drug interactions with azole antifungal drugs.
Side effects in patients receiving more than 7 days of fluconazole, regardless of dose, include the following: nausea, headache, skin rash, vomiting, abdominal pain, and diarrhea. Use of high doses may be limited by nausea. Reversible alopecia may occur with prolonged therapy at 400 mg daily. Rare cases of deaths due to hepatic failure or Stevens-Johnson syndrome have been reported. Fluconazole is teratogenic in rodents and has been associated with skeletal and cardiac deformities in at least 3 infants born to 2 women taking high doses during pregnancy. Fluconazole is a Category C agent that should be avoided during pregnancy unless the potential benefit justifies the possible risk to the fetus.
SUNSCREEN AGENTS

A 23-year-old woman is planning a beach vacation. She asks you for advice on sunscreen agents.
a. What are sunscreens?
Photoprotection from the acute and chronic effects of sun exposure is readily available with sunscreens. The major active ingredients of available sunscreens include chemical agents that absorb incident solar radiation in the UVB and/or UVA ranges and physical agents that contain particulate materials that can block or reflect incident energy and reduce its transmission to the skin (see the Table SUNSCREEN AGENTS). Many of the sunscreens available are mixtures of organic chemical absorbers and particulate physical substances. Ideal sunscreens provide a broad spectrum of protection and are formulations that are photostable and remain intact for sustained periods on the skin. They also should be nonirritating, invisible, and nonstaining to clothing. No single sunscreen ingredient possesses all these desirable properties, but many are quite effective nonetheless.
The major measurement of sunscreen photoprotection is the sun protection factor (SPF), which defines a ratio of the minimal dose of incident sunlight that will produce erythema or redness (sunburn) on skin with the sunscreen in place (protected) and the dose that evokes the same reaction on skin without the sunscreen (unprotected). The SPF provides valuable information regarding UVB protection but is useless in documenting UVA efficacy. In 2007, the FDA proposed a consumer-friendly rating system for UVA products consisting of 1 to 4 stars representing low, medium, high, and highest UVA protection available in an OTC sunscreen product as an indicator of the product’s ability to prevent tanning. The test proposed to determine UVA rating is analogous to the SPF test used to determine the effectiveness of UVB sunscreen products.
Except for total sun avoidance, sunscreens are the best single method of protection from UV-induced damage to the skin.
A 65-year-old woman has a complaint of itching on her arms and legs. Physical examination shows that she has broken skin on her legs from scratching.
a. What are the common causes of itching?
The term pruritus is derived from the Latin prurire, which means “to itch.” Pruritus is a symptom unique to skin that occurs in a multitude of dermatological disorders, including dry skin or xerosis, atopic eczema, urticaria, and infestations. Itching also may be a sign of internal disorders, including malignant neoplasms, chronic renal failure, and hepatobiliary disease. In addition to treating the underlying disorder, a general approach to the treatment of pruritus can be made by classifying pruritus into one of four clinical categories (see Table 48-6).
TABLE 48-6 Agents Used for the Treatment of Pruritus
Pruritoceptive Pruritus: Itch originating in the skin due to inflammation or other cutaneous disease
• Emollients—Repair of barrier function
• Coolants (menthol, camphor, calamine)—Counter-irritants
• Capsaicin—Counter-irritant
• Antihistamines—Inhibit histamine-induced pruritus
• Topical steroids—Direct anti-pruritic and anti-inflammatory effects
• Topical immunomodulators—Anti-inflammatories
• Phototherapy—Reduced mast cell reactivity and anti-inflammatory effects
• Thalidomide—Anti-inflammatory through suppression of excessive tumor necrosis factor-α
Neuropathic Pruritus: Itch due to disease of afferent nerves
• Carbamazepine—Blockade of synaptic transmission and use-dependent sodium channels
• Gabapentin—Suppresses neuronal hyperexcitability by inhibiting voltage-dependent calcium channels
• Topical anesthetics (EMLA, benzocaine, pramoxine)—Inhibit nerve conduction via decreased nerve membrane permeability to sodium
Neurogenic Pruritus: Itch that arises from the nervous system without evidence of neural pathology
• Thalidomide—Central depressant
• Opioid-receptor antagonists (naloxone, naltrexone)—Decrease opioidergic tone
• Tricyclic antidepressants—Decrease pruritus signaling through alteration in neurotransmitter concentrations
• Selective serotonin reuptake inhibitors (SSRIs)—Decrease pruritus signaling through alteration in neurotransmitter concentrations
Psychogenic Pruritus: Itch due to psychological illness
• Anxiolytics (alprazolam, clonazepam, benzodiazepines)—Relieve stress-reactive pruritus
• Antipsychotic agents (chlorpromazine, thioridazine, thiothixene, olanzapine)—Relieve pruritus with impulsive qualities
• Tricyclic antidepressants—Relieve depression and insomnia related to pruritus
• SSRIs—Relieve pruritus with compulsive qualities
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree