Fig. 8.1
(a) Vesicle and bullae formation within the area of erythema (Unpublished slide of a SM veteran under Prof. Balali-Mood’s medical care, taken with permission of the patient). (b) Erythema, erosion and bulla formation of buttocks, intergluteal and thighs after sulfur mustard exposure. (Unpublished slide of a SM veteran under Prof. Balali-Mood’s medical care, taken with permission of the patient)
A positive Nikolsky’s sign usually present in these patients (Momeni et al. 1992; Kehe and Szinicz 2005).
Because all chemical reactions are complete within a few minutes of the agent penetrating the skin, the fluid in the blister caused by SM does not contain active the vesicant (Mellor et al. 1991; Kehe and Szinicz 2005).
Late-onset bullae have seen in 6 % of patients over normal appearing skin after a few days or weeks of injury (Momeni et al. 1992). The anatomical location of the exposed skin is highly important. Bullous lesions are more likely to occur on warm, moist areas such as genitalia, axilla, and areas where tight clothing is worn (Smith et al. 1919; Smith and Dunn 1991) as shown in Fig. 8.1b. Because of their special condition like warmth, moisture and less thickness these area have a lower dermal barrier function (Kehe and Szinicz 2005). Also abundance of hair follicles (Smith et al. 1919; Smith and Dunn 1991), and high density of sweat glands (Mellor et al. 1991) may prepare a suitable condition for skin absorption of SM which makes them as a common site of SM skin injuries.
With blister formation the itching normally diminishes (Sohrabpour 1984; Willems 1989). However, pain and itching are still the main clinical symptoms at this stage. Large blisters usually rupture resulting in erosions, this can also lead to ulceration and full-thickness skin loss, in which secondary infection may follow.
During the first and second days, skin necrosis may occur, leading to worsening of the pain. At the start of the 4th day, eschar formation becomes apparent at the site of necrosis which begins to slough by 4–6 days, leaving a hyperpigmented scar (Mellor et al. 1991).
Superficial blisters and ulcers usually heal within 2 weeks while deep ulcers mostly heal during 4–8 weeks, by leaving a scar. SM-induced ulcers heal more slowly than thermal burns (Mellor et al. 1991), that maybe due to DNA alkylation which results in the reduced proliferation of epidermal cells, particularly in the basal layer and systemic immunosuppression via immune cell damage and death (Bennion and David-Bajar 1994). Moreover, the healing rate differs based on body sites. Blisters which form in the face heal faster usually during a week, whereas blisters of other body sites may require 2–6 weeks and occasionally up to 12 weeks for healing; this is mostly true for the lesions located on the feet. This difference is partly due to abundance of adnexa such as hair follicles in the face (McNamara 1960). Also the healed mustard burns are hypersensitive to mechanical trauma (Chiesman 1944; Mellor et al. 1991).
Regions with severe exposure usually lose their pigments whereas the surrounding areas of lesions having milder injuries, become hyperpigmented (Klehr 1984; Requena et al. 1988; Mellor et al. 1991; Smith et al. 1995). The resulting poikiloderma is a characteristic cutaneous late complication of SM poisoning that may persist for decades (Kehe and Szinicz 2005).
The majority of SM victims are adults, although, in cases that civilians were targeted resulting in child injuries in which the rate of involvement was higher than adults. One of the main reason may be their thinner skin compared to adults (Momeni and Aminjavaheri 1994).
There are some discrepancies in the common signs and symptoms of acute SM skin injuries in different studies which demonstrated in Table 8.1 (Balali-Mood 1984; Moradi et al. 1986; Balali-Mood et al. 1991; Momeni et al. 1992; Naraghi et al. 2005).
Table 8.1
Prevalence of acute and chronic complications of sulfur mustard skin injuries based on the results of different studies
Acute | Chronic | ||
|---|---|---|---|
Complication | Prevalence (range %) | Complication | Prevalence (range %) |
Erythema | 21–84 | Pruritius | 25–95 |
Pruritus | 40–97 | Burning sensation | 10–52 |
Burning sensation | 40–97 | Dry skin | 2–44 |
Vesicle and/or Blister | 15–81 | Cherry angioma | 12–38 |
Ulcer | Up to 71 | Erythematous papules | 0–42 |
Hyper pigmentation | 20–84 | Hyper pigmentation | 6–55 |
Pain | 25–77 | Hypo pigmentation | 0–40 |
Depigmentation | 0–4 | ||
Scar (Atrophic & Hypertrophic) | 2–31 | ||
Hair loss | 0–39 | ||
8.3.2 Delayed and Chronic Skin Complications of SM Exposure
The studies performed in this respect can be categorized into two groups:
8.3.2.1 Delayed Complications in War Veterans
That includes studies on individuals who have been exposed to sulfur mustard usually once in battlefields; which is better to use the term “delayed” or “late” for their complications.
In this group similar to the acute phase, complications may vary depending on the severity of poisoning and other aforementioned factors.
Based on some studies, while the respiratory tract complications of sulfur mustard often intensify and the eye symptoms remain unchanged, the cutaneous complications alleviate over time (Shirazi and Balali-Mood 1988).
The long-term cutaneous complications has been reported in 23 % (Ghassemi-Broumand et al. 2008), 24.5 % (Khateri et al. 2003), 41 % (Balali-Mood 1986) and 90 % (Emadi et al. 2008b) of victims. However, in another study conducted on Iraq-Iran war veterans with severe injuries, skin involvement has been reported in 75 % of the cases (Balali-Mood et al. 2005).
Mild injuries in the acute phase causing limited signs such as eryethma and edema result in complete healing and do not leave any foot prints in the delayed phase (Warthin et al. 1918; Chiesman 1944), while in cases which blisters and ulcers develop; the healing process may lead to pigmentary changes or scar formation (Fig. 8.2a).
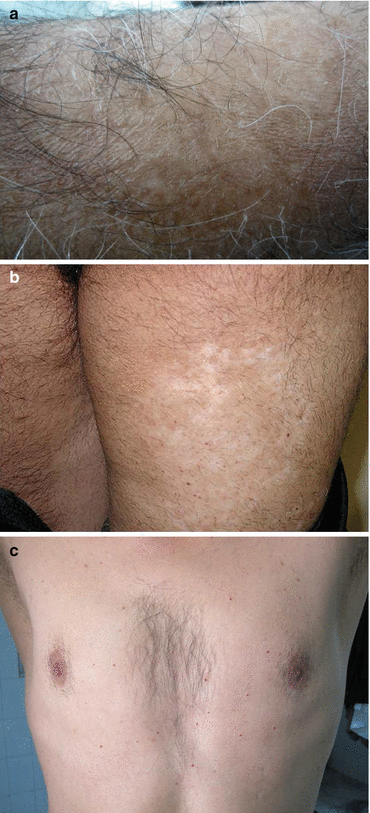

Fig. 8.2
(a) Skin dryness with fine scaling of the forearm (Unpublished slide of a SM veteran under Prof. Balali-Mood’s medical care, taken with permission of the patient). (b) Scaring with hypo and hyperpigmentation of skin on back of the thigh (Unpublished slide of a SM veteran under Dr. Layegh and Dr. Maleki’s medical care, taken with permission of the patient). (c) Multiple and eruptive cherry angioma on the trunk. (Unpublished slide of a SM veteran under Prof. Balali-Mood’s medical care, taken with permission of the patient)
The most common symptom in such patients is itching followed by a burning sensation and dryness of skin which is more common on the extremities, particularly the arms and legs (Fig. 8.2b).
The main cause of itching seems to be skin dryness which is intensified in cold and dry climates and with repeated bathing with hot water and the use of detergent.
The mechanism of long-term dryness of skin has been evaluated in several studies.
In a study the level of skin hydration (SH) and trans-epidermal water loss (TEWL) was measured at four different body locations: forehead, suprasternal, palm and dorsum of hand compared in SM-exposed veterans with the healthy subjects and patients with eczema. The interval between their last hygiene toilets before the measurements was at least 3 h.
Accordingly, although the prevalence of dry skin (xerosis) was significantly higher in the SM-exposed group and in patients with eczema compared to the normal population, interestingly skin hydration in the dorsal and palmar sides of hands and the forehead areas was higher in the SM-exposed group than the non-exposed subjects. In addition, TEWL in SM-exposed subjects was significantly higher than the control group only in the suprasternal region and dorsal side of the hands (Davoudi et al. 2009).
Moreover, regarding the measurement of skin sebum content and elasticity in four areas: forehead, suprasternal, palm and back of the hands, skin sebum was higher in participants who presented with dermatitis and had history of contact with SM than other control groups; the difference was only statistically significant on the forehead (Davoudi et al. 2010).
In another study (Layegh et al. 2015), the skin hydration and sebum content of SM veterans in the flexor and extensor aspects of the forearm and medial and lateral sides of the legs was less than the control group; the difference being significant only for the lateral side of the legs. In the mentioned study, the common sites of dry skin were studied and the interval from the last hygiene toilet was determined as 24 h.
The differences between results of these studies may be due to selection of body location or time interval between the last toilet of the patients and the skin sebum and hydration measurement. The common signs and symptoms seem to vary in different studies as demonstrated in Table 8.1 (Fekri and Janghorbani 1995; Heidari et al. 2000; Moosavi et al. 2001; Toosi et al. 2002; Hefazi et al. 2006; Rezvani et al. 2006; Emadi et al. 2008b; Moin et al. 2009).
They mainly include itching, skin dryness, hyper- and hypopigmentation, poikiloderma and scar formation. Furthermore, multiple erythematous papules may be seen mainly on the trunk and arms of patients that does not match any skin disease (Balali-Mood et al. 2005; Rezvani et al. 2006). Previously injured sites were reported to be sensitive to subsequent mechanical injury and showed recurrent blistering after mild injury (Chiesman 1944; Mellor et al. 1991).
Several studies have reported a higher prevalence of eczema (Mellor et al. 1991; Momeni et al. 1992; Fekri and Janghorbani 1995; Balali-Mood et al. 2005; Emadi et al. 2008b; Moin et al. 2009), hair loss (Fekri and Janghorbani 1995; Toosi et al. 2002; Hefazi et al. 2006), urticaria and angioedem (Fekri and Janghorbani 1992; Rezvani et al. 2006; Emadi et al. 2008b), vitiligo (Fekri and Janghorbani 1992; Emadi et al. 2008b), psoriasis (Emadi et al. 2008b) in SM-exposed patients in comparison to the healthy population.
There are certain differences in various studies about some skin diseases such as acne vulgaris and pityriasis versicolor. Some researchers have reported an increase (Moosavi et al. 2001; Emadi et al. 2008b; Moin et al. 2009) and others a decrease in their incidence (Fekri and Janghorbani 1995) while they have not been addressed in other similar studies. It seems the differences in these results may be due to the severity of injury and the time interval between exposure and the time of study.
Overtime, additional lesions may develop in such patients. Multiple and eruptive cherry angioma has been reported in several studies over 10 years from the exposure, whereas it was not mentioned in the early reports (Firooz et al. 1999; Moosavi et al. 2001; Moradi and Aghaei 2004; Balali-Mood et al. 2005; Maleki et al. 2006; Moin et al. 2009) (Fig. 8.2c). Also there is evidence that veterans with severe itching have a significant less quality of life than patients with milder symptoms (Panahi et al. 2008).
8.3.2.2 Chronic Complications Due to Occupational Exposure
This part includes studies on subjects who have been exposed to sulfur mustard while working in mustard gas factories and have often been under chronic and prolonged contact with small amounts of this material; that is better to use the term “chronic” for their long-term side effects.
Occupational exposure to SM could induce pigmentary changes, skin ulcers and increase the probability of skin cancers (Klehr 1984).
In a study from Japan on 488 former workers of a SM factory, 155 cases were reported with pigmentary disorders in the form of hyperpigmentation and depigmentation as a rain drop even on the covered areas of the body (Sidell 1998).
8.4 SM Carcinogenesis
Based on laboratory studies, SM is a carcinogenic agent and several clinical studies have reported increased rates of lung and skin cancer among workers of SM factories (Wada et al. 1968; Inada et al. 1978; Nishimoto et al. 1983; Easton et al. 1988).
Regarding war veterans, despite several reports indicating a rise in lung and skin cancers, yet the subject is still controversial and requires long-term cohort studies. Carcinoma of the nasopharynx, bronchogenic carcinoma, adenocarcinoma of the stomach, as well as acute myeloblastic and lymphoblastic leukaemia, have been reported in Iranian veterans (Balali-Mood 1992; Ghanei and Vosoghi 2002).
In one study cancer incidence was significantly increased in Iranian war vetrerans exposed to SM. The incidence rate ratio of cancer was 1.81 (95 % CI 1.27–2.56) with hazard ratio of 2.02 (95 % CI 1.41–2.88). Finally, the authors concluded carcinogenesis of SM following acute exposure during war and recommended improvement care programs such as routine screening schemes for exposed veterans (Zafarghandi et al. 2013).
Regarding skin cancer, a study performed on 800 war veterans, skin cancer was diagnosed in 1.1 % which in comparison to the normal population (0.01 %), showed a statistically significant difference. These cancers included BCC, SCC, Bowen’s disease, dermatofibrosarcoma protuberans and Mycosis fungoides which mostly developed at the site of SM-induced scar lesions (Emadi et al. 2008b).
Also a case of Merkel cell carcinoma at the site of SM-induced scar has been reported (Maleki et al. 2008).
Although there are some evidences about carcinogenicity of SM in war veterans till now, but this relationship is uncertain and considering prolonged time needed to elapse for development of skin cancers, a long-term follow-up is recommended.
8.5 Histopathology of Mustard Skin Injuries
The majority of data on the histopathology of mustard skin injuries has been gathered from experimental animal models. Although within several minutes after exposure, mustard fixes to the tissue, histopathological changes are not evident until 30–60 min later and do not complete till 2–3 days after exposure (Bennion and David-Bajar 1994).
The histopathology of SM exposed skin including light microscopy findings and ultrastructural pathology by electron microscopy could be classified in 3 stages; prevesication (4–6 h after exposure); vesication (6–24 h post exposure) and scar (after 1 year). During prevesication, the earliest changes limited to individual basal keratinocytes occur by becoming dyskeratotic and pyknotic. Nuclear chromatin margination, nuclear envelope dilatation, mitochondrial swelling of basal cells, intercellular spaces widening, disqualifying of desmosomes and hemidesmosomes also succeed.
In the vesication stage suprabasal and stratum spinosum cells show nuclear pyknosis, vacuolation in cytoplasm, mitochondrial density and endoplasmic reticulum swelling. These changes lead to microvesicle formation within the lamina lucida of the basement membrane which primarily appears at 12 h post exposure. Then, the microvesicles in lamina lucida are infiltrated with inflammatory cells especially leukocytes, phagocytic cells, cellular debri, degenerating cells and tissue fluid to form blisters and bullae. The presence of large amounts of melanin in all epidermal layers even the horny layer and numerous malanophages filled with coarse melanin granules in the upper dermis have also been described in this stage (Fig. 8.3a–c).
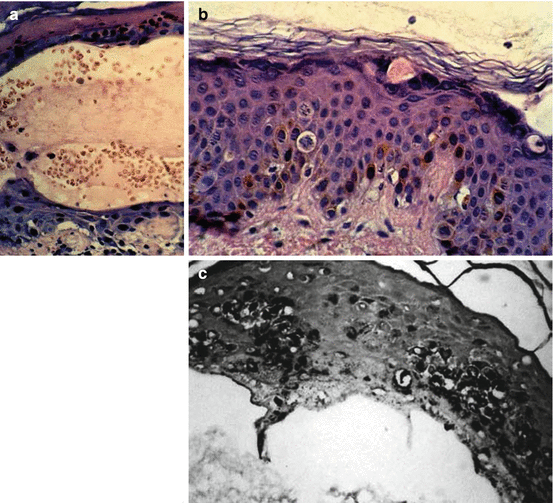

Fig. 8.3
(a) Subepidermal and intraepidermal hemorrhagic blister and also regenerative changes after several days (H&M × 400). (b) Apoptosis/necrosis and mitotic activity in basal layer and layers above it (H&M × 400). (c) Basal hyperpigmentation, increased basilar melanin along with upward transmigration of the melanin (Fontana, × 400) (Reprinted with permission from Naraghi et al., and John Libbey Eurotext publishing)
Finally in SM-induced scars, marked epidermal atrophy, acanthosis with flattened rete ridges, alteration in basal layer pigmentation, perivascular mononuclear infiltrate scattered through the papillary dermis, melanophages in the upper dermis, nonspecific dermal fibrosis, and atrophy of dermal appendages such as sebaceous glands, hair follicles and sweat glands have been reported (Balali-Mood and Hefazi 2006; Emadi et al. 2008a; Emadi et al. 2011; Poursaleh et al. 2012). Hypodermis has been described as normal with no changes in nearly all available reports (Coppens and Roels 1986).
Naraghi et al. have described the histopathologic features of acute cutaneous lesions of 32 Iranian veterans at 5th day of SM exposure, as four distinct patterns: interface dermatitis, vacuolar type and lichenoid type; spongiotic dermatitis and bullous dermatitis with or without acantholysis; pigmentary disorders pattern, increase in epidermal melanization; alteration of dermis/hypodermis, vasculopathy and appendageal inflammatory response; sclerodermoid pattern. Despite description of some specifications related to SM skin injury, they concluded that these findings were compatible with the histopathologic changes in any chemical burns (Naraghi et al. 2005).
8.6 Management of Vesicant Injury
In spite of considerable investigation regarding the treatment of SM damages during the past 20 years, no effective treatment or specific antidote has yet been developed for skin injuries due to SM exposure. As yet, the mainstay of treatment has been symptomatic therapy. The management of SM casualties can be divided into several phases: prophylaxis, decontamination and treatment of lesions (Bennion and David-Bajar 1994).
8.6.1 Prophylaxis
Prophylaxis consists of using protective equipment, avoidance of contaminated areas and destruction of the chemical capability of the enemy (Bennion and David-Bajar 1994). Personal protective clothing includes gas mask, gloves, suit and foot protection. Also for skin areas located at the junction of protective clothing like the wrist, waist, neck and ankle, some topical skin protectants like butyl rubber or polyvinyl chloride gloves and boots can be used (Poursaleh et al. 2012).
8.6.2 Decontamination
SM is infamous for its persistence and ability to adhere to fomites. Mustard casualties and fomites could be decontaminated passively by absorption to inert substances or deactivation and detoxification by chemicals (active decontamination) (Jenner and Graham 2013). The standard way to inactivate mustard compounds includes the US Army’s M13 decontamination kit which contains a dusting pad of fuller’s earth that absorbs liquid SM and the newer one, M258 containing a solution of chloramide and a mixture of phenol, ethanol and sodium hydroxide (Bennion and David-Bajar 1994).
One of the best examples for chemical neutralization is reactive skin decontamination lotion (RSDL), a product that has been approved by the FDA in 2003 and undergone military use by the American forces. It has an efficacy equal to fuller’s earth (Taysse et al. 2007). The solvent portion of this lotion solubilizes chemical weapons away from the skin whereas its oxime component readily reacts with mustards and even nerve agents to produce less toxic products (Sawyer et al. 1991a, b). Treatment of domestic pigs with RSDL, 5 min after exposure to SM eventuated significantly less injury after 3 days (Taysse et al. 2007).
Other chemical agents from this group include sodium thiosulfate, as a reducing agent (Owens and Hatiboglu 1961; Bonadonna and Karnofsky 1965) and 2, 3-dimercapto-propen sulfonic acid (DMPS) which has shown protective effects in mice exposed to SM vapor (Pant et al. 2000).
In the absence of standard kits, washing skin repeatedly with soap and/or shampoo and large amounts of warm water could inactivate large quantities of mustard (Aasted et al. 1987). Beside water, washing with other substances such as oil, gasoline, kerosene and surgical spirits have also been proposed (Jelenko 1974; Gold et al. 1993; Wormser et al. 2002).
If water is not easily available, application of absorbent powders such as grain flours, talcum powder (van Hooidonk et al. 1983), fuller’s earth that is clay-rich soil which has an almost irreversible bond to SM (Chilcott et al. 2001), and activated charcoal or even mechanical scraping could be used. Strong basic solutions like ammonia and lye or chlorinated acids such as sodium hypochlorite especially when used in a proper ratio to SM like 1000:1, and in appropriate concentrations of 0.5 and 4 % solution could effectively hydrolyze SM and may be used to decontaminate fomites (Papirmeister et al. 1985; Bennion and David-Bajar 1994; Wormser et al. 2004).
Vaporized hydrogen peroxide which generally used in industry as a gaseous sterilant has been shown to be an effective SM decontaminant in the presence of ammonia (Wagner et al. 2007).
Creams containing fluorinated cross-linker monomers could also be applied as a decontamination agent (Liu et al. 1999). The rate of skin absorption has reduced by 18-fold after using perfluorinated creams in some cases (Chilcott et al. 2002).
Active ingredients within the cream by actively reacting with SM, can decontaminate it. Because chemical agents could snare in these creams and prevent natural off-gassing, they should be administered with caution. It is to be noted that in cases of vapor exposure to SM, decontamination is not effective (McNamara 1960).
In a study by Vijayaraghavan et al, 20 % of CC2 (N,N′-dichloro-bis [2,4,6-trichlorophenyl] urea) in hyroxypropyl cellulose was reported as a safe chemical substance and a personal SM decontaminant (Vijayaraghavan et al. 2002).
Oral administration of amifostine and ethyl phenyl sulfoxide which is a newly synthetized compound has been reported to be effective as a prophylactic agent against SM toxicity (Kumar et al. 2002). Also the combination therapy of acetaminophen and N-acetylcysteine on human skin fibroblast cells before or concomitant with SM exposure, could reduce its toxicity (Saberi and Zaree Mahmodabady 2009).
8.6.3 Conventional Therapy
Supportive care similar to that performed for severe thermal burns has the principle role in the treatment of SM skin lesions and their related symptoms. The patients must be carefully monitored for limiting associated complications. Extensive damage to the epidermal barrier results in increased fluid loss which could lead to hypovolemia, electrolyte imbalance, renal insufficiency and sepsis.
8.6.4 Management of Acute Skin Lesions
Skin injuries with less than 20 % involvement of body surface area are unlikely to lead to significant complications, essentially due to electrolyte and fluid imbalances (Chan 1987). They could be managed in a non acute care setting such as a dermatology ward.
Topical care is initiated after precisely cleansing and decontaminating the involved areas. Careful daily wound care is essential. Erosions and denuded areas should be monitored for early signs of bacterial infection. Cleansing the wounds and if necessary their debridement is essential to avoid developing infections. Anti- infectious creams such as silver sulfadiazine or mafenide acetate (sulfamylon) should be used to inhibit bacterial colonization and infection of erosions and denuded skins. Topical antibacterial ointments or creams such as bacitracin, neomycin and polymyxin B (Neosporin) could be protective against erosions and bullas and accelerate re-epithelialization (Winton and Salasche 1985). Using biosynthetic dressings such as hydrocolloids and hydrogels could lead to absorption of wound fluids and could induce faster wound healing, accelerate reepithelialization and reduce pain (Eaglstein 1985).
Aspiration and deroofing are the main actions for larger blisters, in order to accelerate the healing process (Graham et al. 2005). Several recent studies have focused on the use of physical debridement of injured tissue via surgical removal followed by skin grafting or application of Xeroform petrolatum and scarlet red ointment dressing (Graham et al. 2000, 2006), by CO2 laser (Graham et al. 1997, 2000) or even dermabrasion (Rice et al. 2000), to enhance the rate of wound healing.
The most common symptoms related to skin damage are pain and itching. Itching could be controlled by antihistamines such as hydroxyzine and doxepin. In areas with severe itching and resistant to antihistamines, topical corticosteroids may be helpful. However, it should be considered that these drugs may slow the healing process. Beside standard analgesics such as codeine, non-steroidal anti-inflammatory drugs such as naproxen can be used for relieving pain and reducing inflammation (Bennion and David-Bajar 1994).
The management of acute skin lesions are summarized in Diagram 8.1.
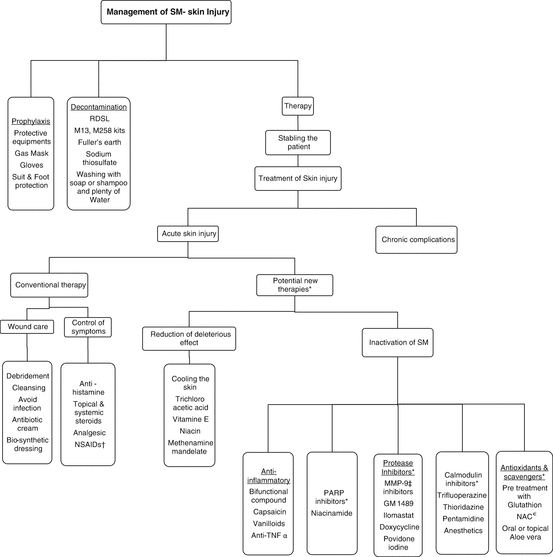

Diagram 8.1
Algorithmic approach to management of Sulfur mustard skin injury (* Some of these therapies have just been shown effective in in-vitro which has been mentioned in the text while their cutaneous administration is not recommended. † Non- steroidal anti-inflammatory drugs. ‡ Matrix metalloproteinase −9. € N-acetyl cysteine)
8.6.5 Management of Chronic Skin Complications
One of the most common late skin complications of SM-exposed individuals in almost all studies is pruritus and/or burning sensation.
Like many other skin disease with pruritis, oral antihistamines, the well- known anti pruritus drugs, have been used for SM-induced pruritus. Previous studies showed efficacy of hydroxyzine 25 mg/day, cetrizine 10 mg/day and doxepin 10 mg/day for 4 weeks in decreasing the severity of such complaints in SM-injured patients (Shohrati et al. 2007b, c). In these studies doxepine had the same efficacy as hydroxyzine taken once a day, but had greater efficacy than cetrizine (Shohrati et al. 2007b, c).
Equal efficacy of doxepin cream to betamethasone was observed in a recent clinical trial which suggests topical doxepin as a potential alternative for controlling the pruritus caused by sulfur mustard in exposed veterans (Panahi et al. 2011).
Based on several clinical trials of Iranian researchers on SM veterans of the Iraq-Iran war (1980–1988), mild to moderate topical corticosteroids are the first line treatment for Pruritus (Vogt et al. 1984; Shohrati et al. 2007a; Panahi et al. 2007, 2008, 2009). Furthermore, in some of these studies, the efficacy of betamethasone cream in controlling SM induced pruritus was compared with other preparations such as capsaicin (Panahi et al. 2008), pimecrolimus (Panahi et al. 2009) doxepin cream (Panahi et al. 2011) and Aloe vera/olive oil cream (Panahi et al. 2012a) which among all, betamethasone cream 0.1 % was superior or as effective as others in reducing chronic skin signs and symptoms caused by sulfur mustard exposure. It is to be considered that although these are effective drugs, their long-term side effects particularly in extensive areas, limit their application.
Calcineurin inhibitors such as tacrolimus and pimecrolimus are available as topical formulations which were first developed for the treatment of atopic dermatitis. They are non-steroidal anti-inflammatory drugs that have been also applied in managing pruritus, burning sensation and skin dryness of SM veterans. The results of a clinical trial showed that they require a longer period of time to achieve the same effects (Panahi et al. 2009), while being free of the many adverse effects of topical corticosteroids. However, other issues such as higher price, age limitations and probability of burning sensation should be considered in the application of these drugs.
In another study, Unna’s boot which is a compression dressing usually made of cotton and impregnated with glycerine, zinc oxide paste and calamine was used and compared with betamethasone ointment for managing SM related pruritus; it showed promising results (Shohrati et al. 2007a). The zinc oxide paste in the Unna’s boot helps ease skin irritation and keeps the area moist.
Capsaicin or Trans −8-methyl-N-vanillyl-6- nonenamide, is a natural alkaloid and the active agent causing spicy taste in hot chili peppers. It is formulated today as a topical cream or lotion which may be used as an adjunct topical analgesic in controlling pain (Lin 2007) and occasionally in intractable pruritus (Lysy et al. 2003).
An investigation revealed significant effects of capsaicin cream 0.025 % (twice a day for 6 weeks) in reducing SM-induced pruritus and skin dryness (Panahi et al. 2008). However, in comparison to the betamethasone cream, it was less effective and less well tolerated.
Until recently, it was thought that capsaicin decreases pain via selective excitation of peripheral un-myelinated afferent C-fibers by releasing of substance-P and finally depleting it; but experimental and clinical studies have shown that depletion of substance P from nociceptors has little, if any, causative role in pain relief. Rather, it acts in the skin through a process best described as ‘defunctionalization’ of nociceptor fibres (Anand and Bley 2011).
Panahi et al in a randomized control trial investigated the anti-inflammatory effects of curcumin in 96 male Iranian veterans who were suffering from chronic SM-induced pruritic skin lesions and concluded that curcumin supplementation effectively alleviate pruritus and improve their QoL (Panahi et al. 2012b).
Aloe vera/olive oil cream was as effective as betamethasone 0.1 % in the treatment of sulfur mustard-induced chronic skin complications and might serve as a promising therapeutic option for the alleviation of symptoms in mustard gas-exposed patients (Panahi et al. 2012a).
Another topical preparation that has been investigated for controlling SM-pruritus is the combination of menthol 1 % and phenol 1 %; it is used twice a day for 6 weeks and has shown significant effects in decreasing pruritus (Panahi et al. 2007). Menthol is an old medicine which contains major monoterpene in the essential oils of some menthe species (Lamiaceae). It is a widely used over-the-counter topical drug for the treatment of pain and its antipruritic effects have been described in several studies (Bromm et al. 1995; Panahi et al. 2007; Haught et al. 2008).
Menthol can relieve itch in some patients by activating the cold-sensitive receptors in the skin that transmit a cool sensation, thereby reducing the perception of itch (Kibbi et al. 1992). In this sense it is similar to capsaicin (Anand 2003).
Another common delayed skin complications in SM-injured patients are skin dryness (xerosis) and eczema. Its treatment is very similar to other causes of skin dryness or eczema. Use of emollients especially the thicker and greasier ones like petrolatum and eucerin that are occlusive and prevent trans-epidermal water loss are preferable. Only mild soap or soapless cleansers such as some pains and body creamy shampoo are recommended while prolonged bathing or excessively warm baths and showers should be avoided (Firooz et al. 2011).
Regarding high concentration of chlorine in some swimming pools which could aggravate skin dryness and itching, we recommend moisturizing the skin after swimming or if available using chlorine free ones.
To date, for abnormal skin pigmentation (hyper or hypopigmentation) due to SM, no effective treatment has been identified (Poursaleh et al. 2012).
The management of chronic skin lesions are summarized in Diagram 8.2.
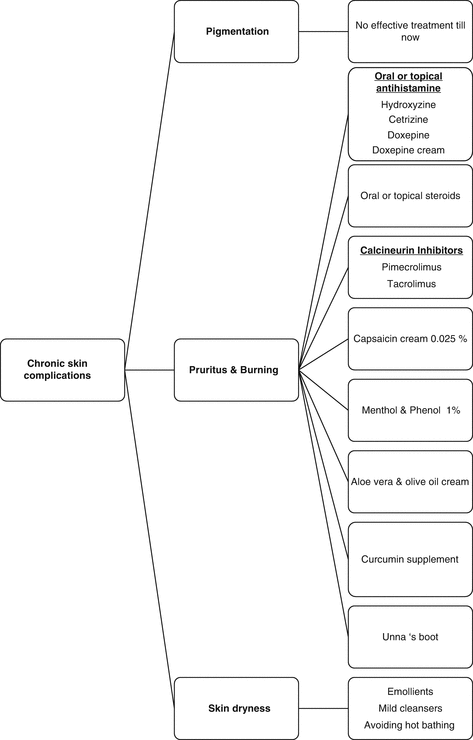

Diagram 8.2
Algorithmic approach to management of chronic skin complications due to sulfur mustard
8.6.6 New Therapies
Recent researches have focused on two main strategies as the principles to finding new treatments for SM injury: (a) Deactivation of SM before causing significant damage to tissues and (b) Reducing SM’s harmful effects.
8.6.6.1 Inactivation of Mustard Compounds
Antioxidants and Scavengers
Skin absorption could continue from free, non-fixed SM compounds or from contaminated fomites, so treatments specifically focusing on inactivating SM may be useful even after the initial exposure. The aim of using these chemical scavengers is to inactivate the free radical forms of SM or the oxygen or nitrogen radicals which result from SM activation (Donald et al. 2009). This type of treatment should be used within minutes to deliver maximum effects, because SM reacts with body tissue within the first minutes of exposure. Most studies that have focused on the therapeutic effects of these scavengers are related to pulmonary exposure or have investigated their ability to reduce leukopenia (Papirmeister et al. 1991) and there is limited research on the skin injury.
There is evidence that thiols or compound containing sulfhydryl groups can decrease the toxic effects of mustard (Walker and Smith 1969; McKinley et al. 1982). Sodium thiosulfate, a potent antioxidant and scavenger, is a thiol containing compound that is currently used to treat cyanide poisoning. It has been used systemically for reducing leukopenia and thrombocytopenia in the treatment with nitrogen mustard prior to exposure (Bonadonna and Karnofsky 1965; McKinley et al. 1982). Nevertheless, it has limited effects on SM cutaneous injuries (Vojvodic et al. 1985; Zhang et al. 1995).
Considering the pivotal role of glutathione in maintaining the intracellular reducing state, pretreatment with glutathione may protect cells against SM toxicity. There are several in vitro studies that have shown pretreatment of various cell lines with glutathione itself or the cysteine precursor; 10 mM L-oxothiazolidine-4-carboylate provides resistance against SM toxicity (Andrew and Lindsay 1998; Amir et al. 1998; Simpson and Lindsay 2005). However, the use of reduced glutathione, once before and twice after SM exposure did not keep mice from toxicity (Kumar et al. 2001). Since cutaneous application of glutathione is difficult, it has not been considered as an appropriate therapeutic agent for this purpose.
N-acetyl cysteine (NAC) is an antioxidant, inducer of glutathione synthesis and redox-active agent. As SM could reduce glutathione in the cell, its recovery may provide increased tissue survival.
In an in vitro study, pre treatment with NAC elevated intracellular glutathione levels and protected the cells against SM exposure (Atkins et al. 2000). It has also been reported to prevent apoptosis in different cell lines such as lymphocytes, neurons and vascular endothelial cells (Dabrowska et al. 1996; Atkins et al. 2000).
In a mouse model, the effect of oral and topical Aloe vera gel on toxicity and skin lesions caused by SM was evaluated. It showed protective results on SM -induced oxidative stress. The results were more prominent for topical administration but were partial for the oral type (Anshoo et al. 2005).
Protease Inhibitors
One of the mechanisms of dermo-epidermal separation in SM-exposed skin with strong evidences in the literature is basement membrane damage by MMPs. Up-regulation of MMP expression especially MMP-9 has been shown in some studies following exposure to SM (Danne et al. 2001; Sabourin et al. 2002; Shakarjian et al. 2006).
There is some evidences supporting the use of protease inhibitors like MMP-9 inhibitors GM 1489 (Gerecke et al. 2005), Ilomastat (Schultz et al. 2004) and doxycycline (Schultz et al. 2004; Guignabert et al. 2005; Lindsay et al. 2008) on human or animal skin cells in vitro.
The beneficial effects of post SM- exposure treatment with iodine have been shown in several studies on rodents. Povidone-iodine ointment is an efficient protective agent against chemical injuries and heat stimuli (Wormser et al. 1997, 2000). Treatment with iodine significantly increases epidermal hyperplasia and reduces inflammation and necrosis.
Both human and animal studies showed that the ointment should be used immediately after SM exposure (Brodsky and Wormser 2007). The proper time for achievement to protective effect of topical iodine application varied in different studies from 15 to 30 min or even up to 60 min (Wormser et al. 2004). The shorter interval between exposure and treatment, the better was the protection achieved (Wormser et al. 2000).
The mechanisms by which iodine take care of the skin against chemical injury such as SM exposure is reduction of collagenolytic activity (Wormser et al. 2002) or may be due to reduced inducible nitric oxide synthase expression (Nyska et al. 2001). Combination of povidone-iodine preparation with anti-inflammatory agents could improve their efficacies and preserve them as a potent antidote against SM skin lesions (Vijayaraghavan et al. 2009).
Although in the majority of these research, reduction of MMP-9 or −2 or decreasing cellular detachment have been reported, their results showed various degrees of impact on SM injury. Therefore, the effect seems to be related to time of administration (before, post or co-exposure to SM) and the method of application as topical or in media.
PARP Inhibitors
It has been supposed that activation of PARP due to SM-induced cellular damage could result in the depletion of cellular NAD+ which finally led to blister formation. So, PARP inhibitors seem to have ability as a useful pretreatment compound to reduce SM induced injuries.
Niacinamide, a precursor for NAD+ synthesis and an inhibitor of PARP, has been demonstrated as an appropriate pretreatment compound to reduce SM-induced skin injury. Pretreatment and post-treatment application of niacinamide alone (Yourick et al. 1992) or in combination with promethazine and indomethacin in a hairless guinea pig skin exposed to SM reduced erythema and microvesicle formation (Yourick et al. 1995).
Calmodulin Antagonists
Calmodulin antagonists and anesthetics were investigated in hairless mice and observed that they may be considered as a choice for treatment of SM-induced skin injuries. Topical pluronic base ointments including lidocaine or pentamide showed beneficial effects when administered immediately after SM exposure on the skin of pig (Kadar et al. 2000). Potent calmodulin antagonist such as trifluoperazine (0.5–1 %) and thioridazine 2 % significantly prevented the development of SM-induced skin lesions. Also pentamidine 10 % showed the similar effect. Anesthetics drugs such as lidocaine and pentobarbital with concentration more than 5 % demonstrated some protective effect (Kim et al. 1996).
Anti-Inflammatory Drugs
Protection effects of steroidal and non steroidal anti inflammatory drugs (NSAIDs) against SM toxicity, given systemically or topically, have been demonstrated the key role of inflammation in SM-skin injury. Although glucocorticoids are effective in reducing edema in the early phase of injury, they do not seem to affect the overall rate of healing. In animal models, using either systemic preparations such as hydrocortisone or dexamethasone or topical steroids like clobetasol prior or after SM exposure resulted in reduction of inflammation (Babin et al. 2000; Casillas et al. 2000; Dachir et al. 2004; Reid et al. 2008). Also the findings on the administration of NSAIDs like indomethacin given from hours before till minutes or hours after SM exposure has been effective in reducing early edema but not the late effects (Babin et al. 2000; Casillas et al. 2000; Kiser et al. 2001). Co- treatment of steroids with NSAIDs has shown more significant results such as less erythema, reduced damage area and occurrence of fewer lesions (Dachir et al. 2004). More recently, bifunctional compounds including NSAIDs (Diclofenac or Ibuprofen) which have the ability to bond with pyridostigmine, were to some extent effective against SM toxicity (Amitai et al. 2005). In addition, there are some other preparations such as capsaicin and its structural analogs known as vanilloids that their anti inflammatory effects including inhibition of edema, leukocyte migration and mast cell degranulation have been previously demonstrated (Brand et al. 1990; Bunker et al. 1991).
These compounds interfere with the release of neuropeptides from sensory fibers and produce desensitization (Campbell et al. 1993). Some studies have shown that pretreatment of skin with vanilloids like olvanil before SM exposure, significantly reduces edema as well as cytokine and chemokine mRNA induction (Casillas et al. 2000; Babin et al. 2000, 2003; Sabourin et al. 2003). Other analogs of capsaicin such as heptyl isovanillamide and homovanillamide have shown similar protective effects against SM (Casbohm et al. 2004).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


