Features
ThinPrep® (TP)
SurePath™ (SP)
Conventional preparation (CP)
Sample collection
Uniform – head of sampling device is discarded
Uniform – head of sampling device is submitted
Uniform – head of sampling device is discarded
Slide preparation
Fully automated
Partially automated
Manual
Number of cells
~50,000
~50,000
~300,000
Slide area
Cells in well-defined 20 mm diameter area
Cells in well-defined 13 mm diameter area
Cells diffusely smeared in 25 × 75 mm area
Image-guided screening
ThinPrep® Imaging System
FocalPoint™ Slide Profiler and FocalPoint Guided Screener
FocalPoint Slide Profiler and FocalPoint Guided Screener
Fixation
Methanol
Ethanol
Alcohol
Cell preservation
Good
Good
Variable
Obscuring factors
None
None
Usually present
Air-drying
None
None
Usually present
HPV testing
Testing from vial (FDA approved)
Testing from vial (not FDA approved but can be validated)
Testing from another sample
Meta-analysis of prospective randomized trials demonstrated no significant difference between CP and LBC in the detection of CIN2/3 lesions
LBC offers a “cleaner” and possibly more efficient cell preparation to review and the ability to perform human papillomavirus (HPV) testing, as well as chlamydia and gonorrhea testing
♦
Both LBC methods have developed location-guided imaging systems designed to present the cytotechnologist with the fields of vision most likely to harbor abnormal cells:
ThinPrep® Imaging System: 22 fields of vision
BD FocalPoint™ Guided Screening Imaging System: 10 fields of vision
The 2014 Bethesda System
Specimen Type
♦
Indicate conventional smear (Pap smear) and liquid-based preparation (Pap test) versus others
Specimen Adequacy
♦
Satisfactory for evaluation (describe presence or absence of endocervical/transformation zone component and any other quality indicators, e.g., partially obscuring blood, inflammation, etc)
♦
Unsatisfactory for evaluation (specify reason)
Specimen rejected/not processed (specify reason)
Specimen processed and examined, but unsatisfactory for evaluation of epithelial abnormality because of (specify reason)
General Categorization (Optional)
Negative for Intraepithelial Lesion or Malignancy
♦
Others: see “Interpretation/Result” (e.g., endometrial cells in a woman aged 45 years or older)
♦
Epithelial cell abnormality: see “Interpretation/Result” (specify “squamous” or “glandular,” as appropriate)
Interpretation/Result
Negative for Intraepithelial Lesion or Malignancy
♦
(When there is no cellular evidence of neoplasia, state this in the general categorization above and/or in the “Interpretation/Result” of the report – whether or not there are organisms or other nonneoplastic findings)
Nonneoplastic Findings (Optional to Report)
♦
Nonneoplastic cellular variations
Squamous metaplasia
Keratotic changes
Tubal metaplasia
Atrophy
Pregnancy-associated changes
♦
Reactive cellular changes associated with:
Inflammation (includes typical repair)
Lymphocytic (follicular) cervicitis
Radiation
Intrauterine contraceptive device (IUD)
Glandular cells status post-hysterectomy
Organisms
♦
Trichomonas vaginalis
♦
Fungal organisms morphologically consistent with Candida spp
♦
Shift in flora suggestive of bacterial vaginosis
♦
Bacteria morphologically consistent with Actinomyces spp
♦
Cellular changes consistent with herpes simplex virus
♦
Cellular changes consistent with cytomegalovirus
Others
♦
Endometrial cells (in a woman aged 45 years or older)
(Also specify if “negative for squamous intraepithelial lesion”)
Epithelial Cell Abnormalities
♦
Squamous cell
Atypical squamous cells
Of undetermined significance (ASC-US)
Cannot exclude HSIL (ASC-H)
♦
Low-grade squamous intraepithelial lesion (LSIL) (encompassing: HPV/mild dysplasia/CIN-1)
♦
High-grade squamous intraepithelial lesion (HSIL) (encompassing: moderate and severe dysplasia, CIS; CIN-2 and CIN-3)
With features suspicious for invasion (if invasion is suspected)
♦
Squamous cell carcinoma
♦
Glandular cell
Atypical
Endocervical cells (NOS or specify in comments)
Endometrial cells (NOS or specify in comments)
Glandular cells (NOS or specify in comments)
Atypical
Endocervical cells, favor neoplastic
Glandular cells, favor neoplastic
♦
Endocervical adenocarcinoma in situ
Adenocarcinoma
Endocervical
Endometrial
Extrauterine
Not otherwise specified (NOS)
Other Malignant Neoplasms (Specify)
Adjunctive Testing
♦
Provide a brief description of the test method(s) and report the result so that it is easily understood by the clinician
♦
Such as small cell carcinoma, papillary serous adenocarcinoma, extrauterine metastatic adenocarcinoma, malignant mixed mullerian tumor, lymphoma, etc
Computer-Assisted Interpretation of Cervical Cytology
♦
If case is examined by an automated device, specify the device and result
Educational Notes and Comments Appended to Cytology Reports (Optional)
♦
Suggestions should be concise and consistent with clinical follow-up guidelines published by professional organizations (references to relevant publications may be included)
♦
Abbreviation: CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; HPV, human papillomavirus; NOS, not otherwise specified; Pap, Papanicolaou
Specimen Adequacy Terminology and Reporting
Adequacy
♦
An adequate cervical Pap test has an estimated minimum of 8,000–12,000 well-visualized and well-preserved squamous cells for conventional preparations (CP) and 5,000 for liquid-based cytology (LBC). The minimum number of squamous cells required for vaginal Pap tests or Pap tests in patients with a history of radiation therapy is 2,000 cells
This minimum cell range should be an estimate aided by published diagrams of representations of microscopic fields with different parameters of microscope objectives/oculars/field number and number of cells
♦
Any specimen with abnormal cells is by definition satisfactory for evaluation regardless of number of cells
♦
Possible quality indicators might include: absence of pertinent clinical information (such as date of last menstrual period, age, etc.), air-drying, poor preservation of cellular material, excessive blood/mucous/exudates, thick cell groups, scant cellularity, or excessive cytolysis
Endocervical/Transformation Zone Component (Figs. 1.1 and 1.2)
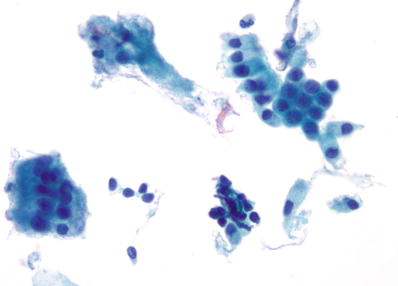
Fig. 1.1.
Satisfactory for evaluation . Endocervical/transformation zone component present. Normal endocervical cells. LBC liquid-based cytology preparation.
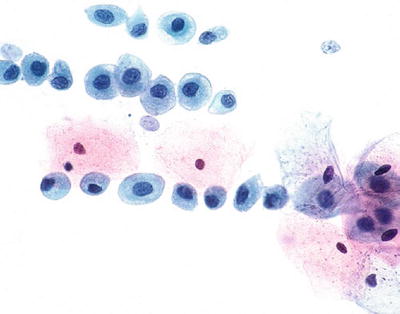
Fig. 1.2.
Satisfactory for evaluation. Endocervical/transformation zone component present. Immature and mature metaplastic cells. CP conventional preparation.
♦
At least 10 well-preserved endocervical and/or squamous metaplastic cells should be observed to report that the endocervical/transformation zone (EC/TZ) was sampled
♦
The presence or absence of EC/TZ component and any other quality indicators should be listed immediately after satisfactory statement based on the above adequacy criteria
♦
According to the 2012 American Society for Colposcopy and Cervical Pathology (ASCCP) management guidelines, women with negative cytology with an absent or insufficient EC/TZ do not necessarily require early repeat testing, especially if the patient has a negative Pap test history:
For women 21–29 years old, routine screening is recommended
For women 30 years or older with no HPV test result, HPV testing is preferred. If HPV test is negative, return to routine screening is recommended. If HPV test is positive, repeat both tests in 1 year. Genotyping for HPV 16/18 is also acceptable. If HPV 16/18 is positive, colposcopy is recommended, and if HPV 16/18 is absent, repeat co-testing in 1 year is recommended
Unsatisfactory Specimen (Fig. 1.3)
Fig. 1.3.
Unsatisfactory for evaluation, obscuring exudate. The specimen is totally obscured by acute inflammatory cells precluding evaluation. Greater than 75% obscuring is considered unsatisfactory if no abnormal cells are identified. CP conventional preparation.
♦
Clarify the laboratory’s role in processing and evaluation of the specimen in the report
Suggested wording to clarify report:
Specimen rejected (not processed) because of the following: specimen not labeled, slide broken, etc
Specimen processed and examined, but is unsatisfactory for evaluation of epithelial abnormality because of obscuring blood, inflammation (greater than 75% of the cells are obscured), etc
♦
Additional comments or recommendations may be suggested as appropriate
Suggested wording to clarify report:
An excessively bloody or inflamed Pap test may hinder the screener’s ability to detect an underlying abnormality and a repeat examination/evaluation is suggested
♦
For LBC specimens that are heavily contaminated with blood, glacial acetic acid (GAA) is employed in order to lyse red blood cells and facilitate interpretation:
Most studies have shown that GAA pretreatment has little effect on commercial HPV tests
♦
Lubricants containing carboners can significantly decrease specimen adequacy and should be avoided when collecting LBC Pap tests. An unusual increase in unsatisfactory Pap tests should warrant notification of the clinicians to determine the cause and whether there has been a change in lubricant
♦
Low squamous cellularity is the most common cause of unsatisfactory Pap tests. Most of these patients are elderly and many have a history of cancer treatment or hysterectomy
♦
A significantly high abnormal follow-up rate is observed in patients with unsatisfactory Pap tests, emphasizing the importance of identifying and following these patients as recommended
♦
The ASCCP 2012 management guidelines:
For an unsatisfactory cytology result and no, unknown, or negative HPV test result, repeat cytology in 2–4 months is recommended. Triage using HPV testing is not recommended
Treatment to resolve atrophy or obscuring inflammation when a known etiology is present is acceptable
For women 30 years or older, with unsatisfactory Pap test and a positive co-tested HPV result, repeat cytology in 2–4 months or colposcopy is acceptable
Colposcopy is recommended for women with two consecutive unsatisfactory Pap tests
Organisms
Trichomonas vaginalis (Fig. 1.4)
Fig. 1.4.
Trichomonas vaginalis . Pear-shaped organisms with eccentrically located nucleus and granular cytoplasm. LBC liquid-based cytology preparation.
♦
Approximately 25% of women are carriers of Trichomonas vaginalis. Trichomonas vaginalis often coexists with Leptothrix and other coccoid bacteria. The organisms are small, “pear or kite-shaped,” and faintly stained with small, oval, eccentric, and pale nuclei and red cytoplasmic granules. Rare flagella may be observed in LBC. “Cannonballs” representing adherence of neutrophils to squamous cells may be observed. The squamous cells may show reactive small perinuclear clearing and cytoplasmic vacuolization, polychromasia, or a “moth-eaten” appearance. Granular debris and inflammation are usually present in the background
Fungal Organisms Morphologically Consistent with Candida Species (Fig. 1.5)
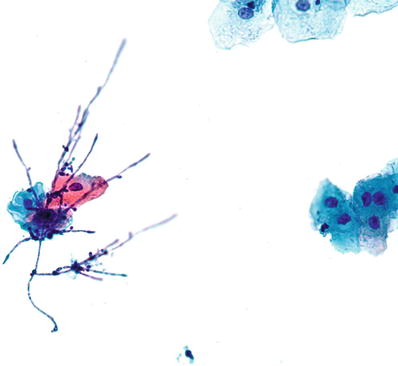
Fig. 1.5.
Fungal organisms morphologically consistent with Candida species. Pseudohyphae and yeast forms are present. LBC liquid-based cytology preparation.
♦
Approximately 10% of females are carriers of Candida organisms. The incidence of Candida infection increases with pregnancy, oral contraceptive use, and diabetes. The organisms are present as yeast forms with long pseudohyphae. “Spearing” of epithelial cells by the pseudohyphae may be observed. Inflammatory cells are generally present in the background
♦
Candida (Torulopsis) glabrata lack the pseudohyphae observed in other Candida species, but may be difficult to separate on Pap test C. glabrata is primarily nonpathogenic except in immunocompromised individuals, where it may rarely be a highly opportunistic pathogen of the urogenital tract and bloodstream
Shift in Flora Suggestive of Bacterial Vaginosis (Fig. 1.6)
Fig. 1.6.
Shift in flora suggestive of bacterial vaginosis. Clue cells with coccobacilli covering the cytoplasm are present. LBC liquid-based cytology preparation.
♦
Bacterial vaginosis occurs in 10–30% of the general population. Patients have exponentially more anaerobes per ml of vaginal fluid than normal. The etiologic agents for bacterial vaginosis include Gardnerella vaginalis, anaerobic lactobacilli, Bacteroides species, and Mobiluncus species. G. vaginalis (haemophilus-corynebacterium-vaginalis) may be cultured in 30–50% of asymptomatic women
♦
A combination of Pap test, wet prep, vaginal pH, and “Whiff” test on KOH preparation (positive in symptomatic women) can establish the diagnosis. The organisms are gram-variable bacilli, including numerous coccobacilli, curved bacilli, or mixed organisms imparting a “filmy” appearance to the preparation. Lactobacilli are absent. “Clue cells” refer to the presence of squamous cells covered by adherent, small, and uniformly spaced coccobacilli. This finding in Pap test alone is neither specific nor sufficient for the diagnosis of bacterial vaginosis
Bacteria Morphologically Consistent with Actinomyces (Fig. 1.7)
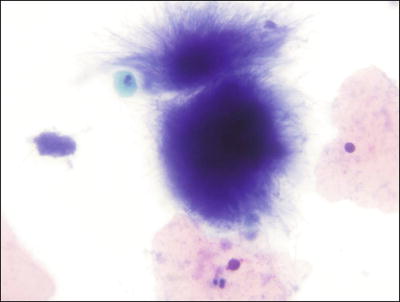
Fig. 1.7.
Bacteria morphologically consistent with Actinomyces species. A cluster of thin filamentous bacilli are present. LBC liquid-based cytology preparation.
♦
Actinomyces organisms are gram-positive filamentous bacteria. They are associated with the use of intrauterine devices (IUD) and vaginal pessaries. Actinomyces organisms are recognized by the presence of isolated tangled aggregates of long basophilic filamentous structures with a radiating pattern. In addition to this morphology, the organisms may be arranged in a horizontal array of filamentous structures along a central dense core, presumably representing the IUD string
Cellular Changes Consistent with Herpes Simplex Virus (Fig. 1.8)
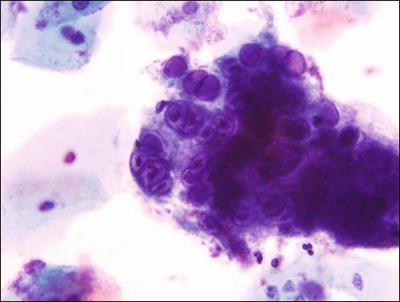
Fig. 1.8.
Cellular changes consistent with herpes simplex virus . Multinucleated giant cell with molding, ground-glass nuclei, and intranuclear inclusions LBC liquid-based cytology preparation.
♦
Eighty percent of exposed females develop herpes simplex virus (HSV) infection following exposure and the recurrence rate is 60%. Herpetic infection is characterized by the presence of multinucleation, molding of nuclei, ground-glass nuclei, margination of chromatin, and eosinophilic intranuclear inclusions. Type I (oral) and type II (genital) herpes, as well as primary and secondary infections, cannot be distinguished cytologically
Chlamydia trachomatis
♦
Intracytoplasmic vacuoles containing eosinophilic dots (elementary particles) are not specific for C. trachomatis as they often represent mucin or other vacuoles. C. trachomatis may be associated with follicular cervicitis especially in younger patients. The Pap test has no role in the diagnosis
Lactobacillus acidophilus (Döderlein Bacilli)
♦
Lactobacillus represent a heterogeneous group of bacilli whose function is to maintain an acidic vaginal pH (3.5–4.5). They are the only species of bacteria that are capable of causing cytolysis (dissolution of cytoplasm of squamous cells) by hydrolyzing intracytoplasmic glycogen
Leptothrix
♦
Leptothrix are filamentous negative rods with or without branching. They are often associated with the presence of another organism (Trichomonas, Candida, etc.)
Molluscum Contagiosum (Pox Virus)
♦
Molluscum contagiosum is characterized by the presence of large cells with eosinophilic intracytoplasmic inclusions (Henderson-Patterson bodies) and pyknotic degenerative nuclei
Enterobius vermicularis (Pinworm)
♦
Enterobius vermicularis has ovoid-shaped eggs with a double-walled shell which is flattened on one side
Entamoeba histolytica
♦
Entamoeba histolytica organisms are large trophozoites with large nuclei and a dot-like central karyosome. Their cytoplasm is vacuolated and contains ingested RBCs
Cytomegalovirus (CMV)
♦
Cytomegalovirus infection in immunocompetent women is usually transient and asymptomatic. The infected cells are enlarged with a solitary basophilic intranuclear inclusion surrounded by a halo. Intracytoplasmic small granular inclusions may also be observed
Contaminants
♦
Alternaria . Air-borne contaminant fungi that have short yellow-brown conidiospores with transversely and longitudinally septate macroconidia (snow shoe-like) (Fig. 1.9)


Fig. 1.9.
Alternaria. Brown macroconidia with transverse and longitudinal septa. LBC liquid-based cytology preparation.
♦
Pollen. Air-borne contaminate that is oval to round with smooth, refractile borders and no internal structure (Fig. 1.10)
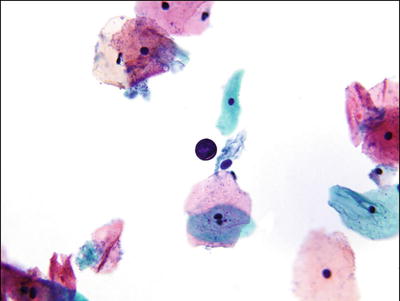

Fig. 1.10.
Pollen. Round granule with smooth refractile borders. LBC liquid-based cytology preparation.
♦
Vegetable cells. Vegetable cells have dense cell walls and structureless nuclei. They may be observed in patients with rectovaginal fistulas along with goblet cells, inflammation, and necrotic debris
♦
Graphite–pencil markings
♦
Lubricant jelly. Not recommended for gynecologic examination prior to Pap test
♦
Cotton, cardboard, and tampon fibers
♦
Trichome. “Octopus-like” or star-shaped structure derived from leaves of arrow-wood plant
♦
Carpet beetle parts. Arrow-shaped structures that may have origin from tampon or gauze pads
♦
Cockleburs. Associated with IUDs, oral contraceptive use, and second half of pregnancy. They are related to cellular degeneration and composed of nonimmune glycoprotein, lipid, and calcium. Cytologically, they are identified as refractile crystalline rays surrounded by histiocytes. They have no clinical significance (Fig. 1.11)
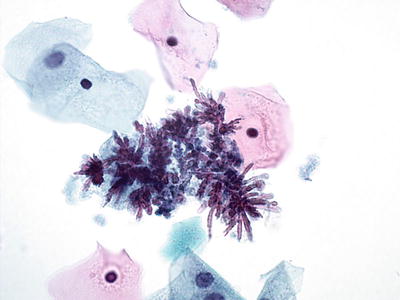

Fig. 1.11.
Cockleburs. Refractile crystalline rays surrounded by histiocytes. LBC liquid-based cytology preparation.
♦
Hematoidin crystals. Composed of bile-like pigment formed from hypoxic tissue, indicative of previous hemorrhage, but does not contain iron. Cytologically, they have finer crystalline rays than cockleburs with variable shapes
♦
Talc particles
♦
Ferning. Represents arborizing palm leaves-like pattern of cervical mucus that occurs at ovulation
♦
“Cornflaking.” A refractile brown processing artifact representing air-trapping between the squamous cells and the cover slip. It can be resolved by recoverslipping (Fig. 1.12)
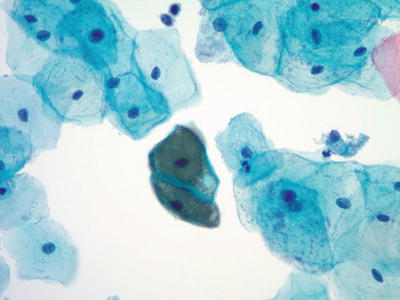

Fig. 1.12.
“Cornflaking.” Brown refractile artifact overlying squamous cells. LBC liquid-based cytology preparation.
♦
Sperm. May be identified in the Pap test for a few days after intercourse. This information is not to be included in the report. Any inquiry should have prompt referral of the slide through the appropriate legal channels
Reactive Changes
Typical Repair (Figs. 1.13 and 1.14)
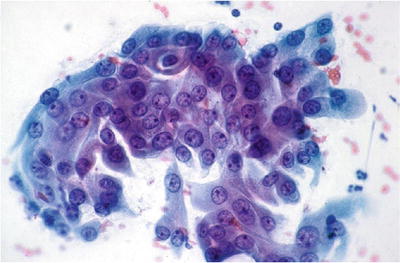
Fig. 1.13.
Reactive cellular changes associated with inflammation. Sheet of squamous cells with distinct borders, abundant cytoplasm, enlarged uniformly round nuclei, and nucleoli. Isolated atypical cells are not observed. CP conventional preparation.
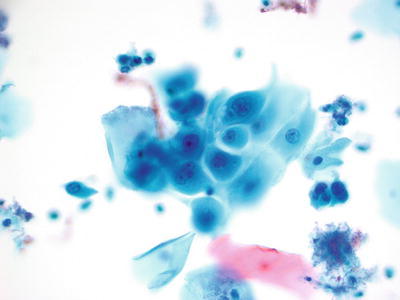
Fig. 1.14.
Reactive cellular changes . Flat sheet of squamous cells with slightly enlarged uniform nuclei, abundant cytoplasm, and nucleoli. Polymorphonuclear leukocytes are present in the background. LBC liquid-based cytology preparation.
Cytologic Features
♦
Characterized by the presence of flat, cohesive sheets of squamous or glandular cells with cellular streaming and pseudopodia (peripheral cytoplasmic projections). No single cells and no tumor diathesis are present
♦
The nuclei are enlarged with fine chromatin and smooth nuclear contours. There are prominent nucleoli that are often multiple and regular
Differential Diagnosis
♦
Squamous cell carcinoma (SCC) presents as single abnormal cells. Tumor cells have an irregular chromatin distribution and multiple irregular nucleoli. A tumor diathesis is present
♦
Acantholytic cells in pemphigus vulgaris may be observed in vaginal smears. Isolated single cells are referred to as tombstone cells. Correlation with clinical history is essential for accurate interpretation of cells derived from pemphigus vulgaris
Radiation Effect (Figs. 1.15 and 1.16)
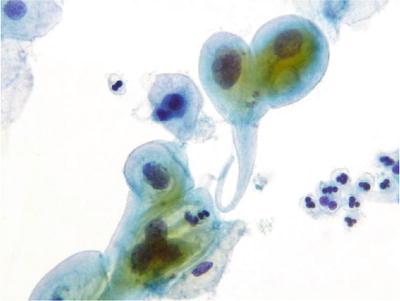
Fig. 1.15.
Reactive cellular changes associated with radiation effect. Large intermediate and metaplastic cells with enlarged nuclei and abundant cytoplasm. Note the cytoplasmic polychromatic staining and vacuolization. LBC liquid-based cytology preparation.
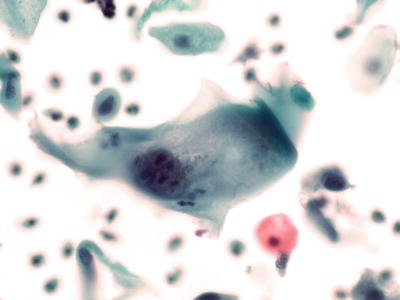
Fig. 1.16.
Reactive cellular changes associated with radiation effect. LBC liquid-based cytology preparation.
Cytologic Features
♦
Cellular enlargement (macrocytosis) and nuclear enlargement, but the nuclear/cytoplasmic (N:C) ratio remains normal. There are nuclear and cytoplasmic vacuoles present with reactive perinuclear halos. Cellular chromatin is finely granular or degenerative (“smudged”). Karyorrhexis and karyopyknosis are observed
♦
Binucleation and multinucleation and micro-and macronucleoli are typical of radiated cells. Large bizarre cells with polychromasia, peripheral cytoplasmic projections (pseudopodia), and cytophagocytosis including intracytoplasmic neutrophils are observed
Intrauterine Device (IUD)-Associated Changes (Fig. 1.17)
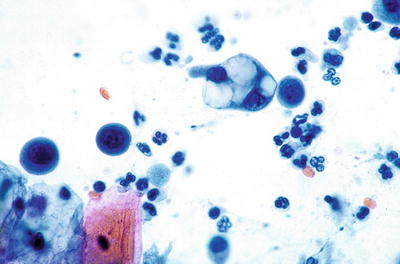
Fig. 1.17.
Reactive cellular changes associated with intrauterine contraceptive device. Glandular cells with cytoplasmic vacuoles displacing the nuclei. LBC liquid-based cytology preparation.
Cytologic Features
♦
Small clusters of hypersecretory endocervical cells with abundant cytoplasm, large cytoplasmic vacuoles (bubblegum cytoplasm), and distinct cell borders
♦
Nuclei are large and uniform and may contain prominent nucleoli. Inflammatory/reparative squamous changes may be present
♦
The background is generally clean or inflammatory. Actinomycotic colonies and calcified debris may be observed
Differential Diagnosis
♦
Adenocarcinoma of endometrium occurs in older patients (postmenopausal) and is characterized by the presence of many abnormal cells with an irregular chromatin pattern and associated tumor diathesis
Atrophy (Figs. 1.18 and 1.19)
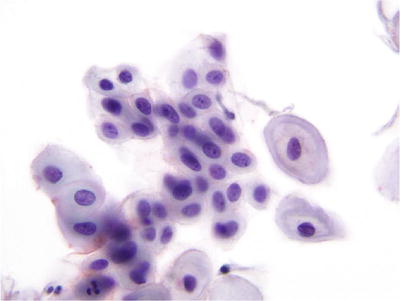
Fig. 1.18.
Negative for intraepithelial lesion or malignancy. Atrophy. Maturation is not observed. Smear consists predominantly of parabasal cells. LBC liquid-based cytology preparation.
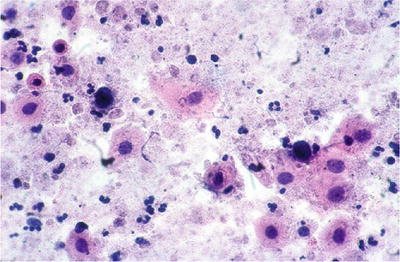
Fig. 1.19.
Negative for intraepithelial lesion or malignancy. Atrophy. Granular background, basal cells, and “blue blobs”. CP conventional preparation.
♦
Generally observed in older women or postpartum
Cytologic Features
♦
Predominance of basal and parabasal cells, either as single cells or in tissue-like sheets. The borders of cell groups are smooth and defined
♦
Atrophic cells show generalized and mild nuclear enlargement with smooth nuclear contours. The chromatin usually appears fine and uniform. No mitotic figures or apoptosis are noted
♦
Air-drying and degeneration may result in autolysis (bare nuclei), orangophilic change in the cytoplasm (pseudoparakeratosis), and a granular background. These changes are more common in CP than in LBC. These background changes can be similar to the tumor diathesis seen in SCC and distinguished only by the absence of malignant cells in atrophy
♦
“Blue blobs” which represent inspissated mucus or nuclear material are often seen in atrophic smears and may mimic atypical cells
Hyperkeratosis (HK )
♦
Often represents a nonspecific reaction to chronic cervicitis, uterine prolapse, previous biopsy, cryosurgery, or instrumentation or as a manifestation of in utero diethylstilbestrol (DES) exposure
♦
Approximately 10% may represent a surface reaction overlying squamous intraepithelial lesion (SIL) or a response to persistent or recurrent disease in patients with prior biopsy or cytology-proven SIL
♦
The presence during pregnancy suggests ruptured fetal membranes
♦
Rare, isolated anucleated cells often represents contaminants from vulva or vagina
Cytologic Features
♦
Clusters or groups of anucleate and granular superficial polygonal squamous cells
Parakeratosis (PK) (Fig. 1.20)
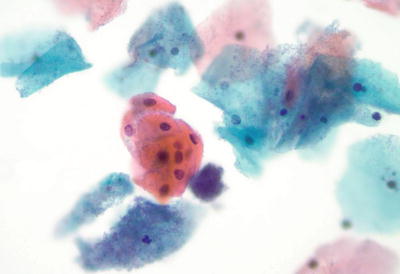
Fig. 1.20.
Reactive cellular changes. Parakeratosis. Superficial squamous cells with uniform, pyknotic nuclei. LBC liquid-based cytology preparation.
♦
Similar to hyperkeratosis, parakeratosis represents a reactive surface process due to chronic irritation, but may be seen with cervical dysplasia
♦
Persistence of parakeratosis without a known etiology may warrant further investigation to exclude an associated SIL
Cytologic Features
♦
Isolated or loose sheets of superficial squamous cells with dense orangophilic cytoplasm and small uniform pyknotic nuclei
Differential Diagnosis
♦
Degeneration can cause a “pseudoparakeratosis” with pyknotic nuclei and cytoplasmic eosinophilia in cases of atrophy or severe inflammation. It is associated with other signs of degeneration, such as karyorrhexis, vacuolization, and cytolysis
Chronic Follicular (Lymphocytic) Cervicitis (Fig. 1.21)
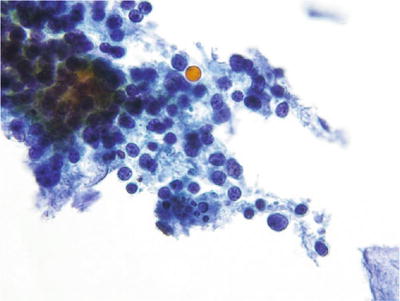
Fig. 1.21.
Reactive cellular changes. Chronic lymphocytic cervicitis. Polymorphous population of lymphoid cells with tingible body macrophages. LBC liquid-based cytology preparation.
♦
Approximately 50% of cases are associated with Chlamydia, but it can also be commonly seen in postmenopausal atrophic specimens
Cytologic Features
♦
Polymorphous population of mature lymphocytes, plasma cells, and histiocytes with characteristic “tingible” body macrophages. Reactive glandular and squamous changes may be observed
Folic Acid Deficiency
Cytologic Features
♦
Similar cytologic changes to those of early radiation effect, including nuclear and cellular enlargement, nuclear enfolding, and multinucleation. The nuclei are hyperchromatic with smooth contours. Cytoplasmic vacuolization and polychromasia are usually observed
Navicular Cells
♦
Observed in pregnancy, late in the menstrual cycle, and in the setting of high progesterone medications
Cytologic Features
♦
Boat-like intermediate cells with ecto-endoplasmic differentiation and glycogen, which may have a yellow hue with Pap stain
Decidual Cells (Figs. 1.22, 1.23, and 1.24)
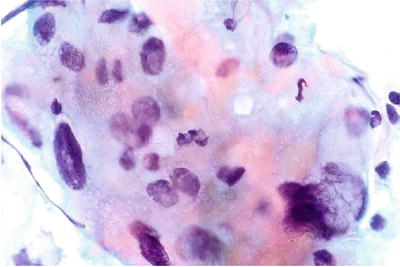
Fig. 1.22.
Decidua. These cells were observed on a Pap smear from a pregnant woman. They show abundant eosinophilic to amphophilic cytoplasm and enlarged nuclei with smudged chromatin pattern. CP conventional preparation.
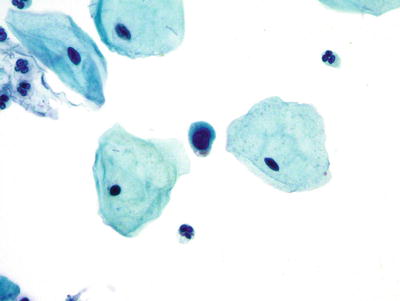
Fig. 1.23.
Decidua. These cells show a high nuclear to cytoplasmic ratio and cytomorphologically mimic ASC-H. LBC liquid-based cytology preparation.
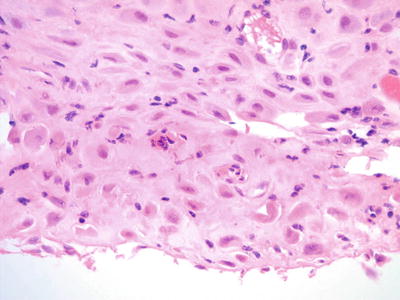
Fig. 1.24.
Decidua. From same patient as Fig 1.23 demonstrating round cells with abundant cytoplasm. There are occasional cells with high nuclear to cytoplasmic ratio (histology, H&E).
♦
May be seen in Pap tests from pregnant women and women on birth control pills or progesterone therapeutic agents. Clinical history is important for accurate interpretation
Cytologic Features
♦
Isolated cells or loose sheets of large, polygonal, or round cells with abundant granular, eosinophilic, or amphophilic cytoplasm. Slight to moderate nuclear enlargement is present, but the N:C ratio usually remains low. The nuclei are round with vesicular or smudged chromatin and degenerative changes. Nucleoli may be prominent
♦
Rarely, decidual cells can mimic squamous and glandular abnormalities and have high N:C ratio (Fig. 1.23)
Trophoblasts
♦
Rarely seen in normal pregnancy
Presence may indicate threatened abortion when observed in the first trimester of pregnancy (6% of patients)
Partial premature separation of the placenta should be suspected when trophoblasts are observed in the third trimester
Retained placental tissue should be suspected when trophoblasts are observed 4 weeks after termination of pregnancy
Cytologic Features
♦
Syncytiotrophoblasts have abundant cyanophilic cytoplasm and distinct cell borders. Their multiple nuclei (20+) are round to oval with nuclear overlapping but without molding. The chromatin is finely granular and evenly distributed with inconspicuous nucleoli
♦
Cytotrophoblasts are large cells with uniformly amphophilic cytoplasm and large irregular nuclei that may be lobulated and vacuolated
Squamous Cell Abnormalities
Atypical Squamous Cells (ASC)
Definition
♦
Cytologic changes suggestive of SIL that are quantitatively or qualitatively insufficient for a definitive interpretation. Atypical squamous cell (ASC) does not represent a single biologic entity. ASC is subdivided into two categories: ASC of undetermined significance and ASC cannot exclude HSIL
♦
The category of ASC is by far the most commonly reported abnormal cervical cytology interpretation. The ASC category was developed to designate the interpretation of an entire specimen, not individual cells, because atypia in individual cells remains highly subjective with variable interpretation
Atypical Squamous Cells of Undetermined Significance (ASC-US) (Figs. 1.25, 1.26, and 1.27)
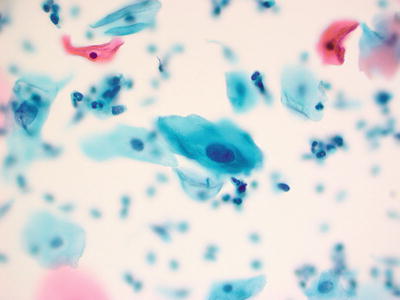
Fig. 1.25.
ASC-US . Atypical intermediate squamous cells with round hyperchromatic nuclei that are at 2.5–3 times the size of the adjacent normal intermediate cell nuclei. LBC liquid-based cytology preparation.
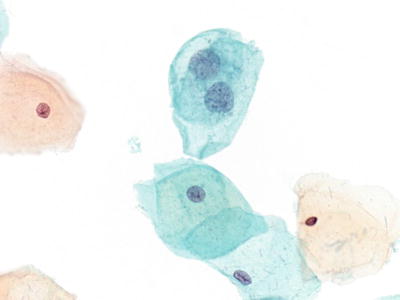
Fig. 1.26.
ASC-US. Intermediate cells with nuclear enlargement. No inflammation is present in the background. LBC liquid-based cytology preparation.
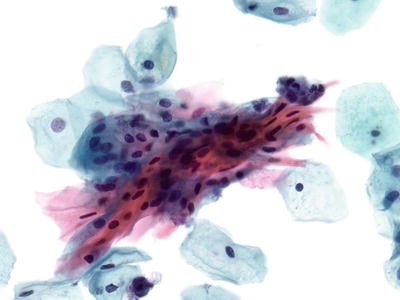
Fig. 1.27.
ASC-US . Atypical parakeratosis. Spindled and slightly pleomorphic orangophilic parakeratotic cells with mild nuclear enlargement. LBC liquid-based cytology preparation.
♦
Represents greater than 90% of all ASC. The average reporting rate is 4.5–5.0% of Pap tests
♦
The ASC-US-LSIL Triage Study (ALTS) trial showed that approximately 50% of patients with ASC-US have oncogenic high-risk HPV (HR-HPV) types
Cytologic Features
♦
ASC-US may manifest a wide range of cytologic features. Designation by cell type of origin is not used; however, these are listed below to assure recognition of ASC-US and to separate it from other, often overlapping, manifestations of reactive cells or more advanced cytologic epithelial abnormalities:
ASC-US involving intermediate or superficial squamous cells represents the most common pattern (Figs. 1.25 and 1.26). The cells are usually isolated and few in number. Nuclear size is 2½–3× size of an intermediate cell nucleus (70–120 μm2). There is a slight increase in N:C ratio. Minimal nuclear hyperchromasia, irregularity, clumping, smudging, or multinucleation may be observed. ASC-US cells do not include inflammatory or reparative/reactive “atypia”
ASC-US cells originating from squamous metaplastic cells have an enlarged nuclei 2× size of the nucleus of a normal immature squamous metaplastic cell. The cytoplasm distinguishes the nuclei as metaplastic; it is less abundant and more cyanophilic and dense. Additionally, the cells are more round with an N:C ratio higher than intermediate or superficial squamous cells
ASC-US may be used when atypical repair is present. Atypical repair has all the features of typical repair in addition to concerning cytologic features, such as cellular overlap, slight lack of cohesion, loss of nuclear polarity, anisonucleosis, and irregularity of nuclear contour. Prominent single or multiple nucleoli are present, as well as mitotic figures and an inflammatory background. If the “atypia” described above is prominent and concerning for HSIL or a more advanced lesion, then atypical repair can be considered under the ASC-H category
ASC-US may be seen in a background of atrophy. Normal atrophic specimens show nuclear enlargement with concomitant hyperchromasia, but if there is marked irregularities in cell shape, nuclear contours, or chromatin distribution, ASC-US may be reported
ASC-US can be used in the setting of atypical parakeratosis (Fig. 1.27). These cells demonstrate the cytologic features seen in parakeratosis except the nuclei are enlarged and more pleomorphic with irregular nuclear borders
Cells with equivocal changes for HPV are placed in the ASC-US category. These cells manifest partial koilocytes or perinuclear halos without nuclear abnormalities. Bi- or multinucleation may be observed
Differential Diagnosis
♦
CP smears may manifest drying artifact that may cause the nuclei to appear larger and washed-out, thus simulating ASC-US. This phenomenon has been almost eliminated by LBC
♦
LSIL shows more marked nuclear enlargement (greater than 3× the size of an intermediate squamous cell nucleus), hyperchromasia, and irregularities of nuclear contour. HPV cytopathic effect is synonymous with SIL
Atypical Squamous Cells, Cannot Exclude HSIL (ASC-H) (Figs. 1.28 and 1.29)
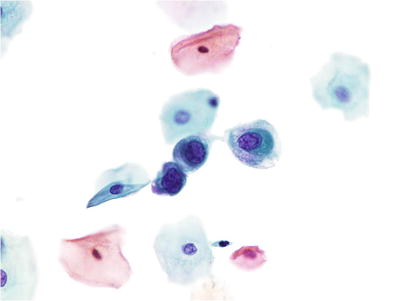
Fig. 1.28.
ASC-H. Atypical immature metaplastic cells with enlarged nuclei and nuclear irregularity. Only rare atypical cells were present. The findings are not diagnostic of HSIL. LBC liquid-based cytology preparation.
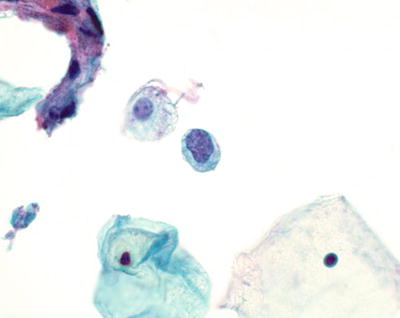
Fig. 1.29.
ASC-H . Atypical immature metaplastic cells. LBC liquid-based cytology preparation.
♦
Defined as having cytologic changes that are suggestive of high-grade squamous intraepithelial lesion (HSIL), but lack ample cytomorphologic features for definitive interpretation
♦
Constitutes approximately 5% of all ASC. The median reporting rate is 0.2–0.3% of Pap tests
♦
The association of ASC-H with underlying CIN2 and CIN3 (30–40%) is lower than for HSIL, but sufficiently higher than for ASC-US. This warrants different clinical management recommendations for ASC-H compared to ASC-US. A few cases of ASC-H may have invasive carcinoma on biopsy (2–3%)
♦
The ALTS trial noted that approximately 70–80% of ASC-H cases have oncogenic HR-HPV types
Cytologic Features
♦
ASC-H may be observed as “atypical immature metaplastic cells.” These occur as small single cells or in tight clusters of usually less than 10 cells. These cells resemble normal metaplastic cells with amphophilic cytoplasm and high N:C ratio but have a slightly larger nuclear size (1.5–2.5× that of normal metaplastic cell nuclei) with nuclear irregularities and mildly hyperchromasia
♦
ASC-H can be used for loose clusters of cells demonstrating markedly atypical repair if the N:C ratio is high and there is nuclear pleomorphism present, raising the possibility of SIL or carcinoma in the differential diagnosis
♦
An interpretation of ASC-H may be rendered for thick tissue fragments or hyperchromatic crowded groups (see section below). The cells show loss of polarity or are difficult to completely visualize, but raise the possibility of HSIL
♦
ASC-H can also be used for rare isolated atypical small cells that do not show features of immature metaplastic cells. These cells demonstrate high N:C ratio, nuclear irregularities or grooves, and hyperchromasia, but without typical cytoplasmic changes seen in metaplastic cells
Differential Diagnosis
♦
ASC-US cells are usually mature squamous cells with low N:C ratio
♦
Immature metaplastic cells have regular round nuclei and uniform finely granular chromatin
♦
HSIL cells are usually more numerous, often occurring in syncytial aggregates, and demonstrate more pronounced nuclear hyperchromasia, nuclear irregularities, and coarse granular chromatin
♦
Endometrial cells are smaller, exfoliate in three-dimensional clusters, and have an eccentric nucleus with finely granular chromatin
♦
Histiocytes have foamy cytoplasm, bean-shaped nuclei, and low N:C ratio
Squamous Intraepithelial Lesions (SIL )
♦
Encompasses a spectrum of noninvasive cervical epithelial abnormalities traditionally classified as flat condyloma, dysplasia/carcinoma in situ, and cervical intraepithelial neoplasia (CIN):
Low-grade SIL (LSIL) includes the cellular changes associated with HPV cytopathic effect and mild dysplasia (CIN1)
High-grade SIL (HSIL) encompasses moderate dysplasia, severe dysplasia (CIN2, 3), and carcinoma in situ (CIS)
Low-Grade Squamous Intraepithelial Lesion (LSIL) (Figs. 1.30, 1.31, and 1.32)
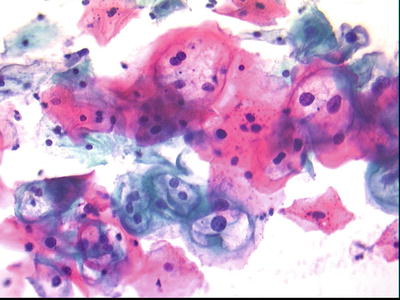
Fig. 1.30.
LSIL Intermediate squamous cells with mild nuclear enlargement and nuclear contour irregularities. Perinuclear halos consistent with HPV effect are present. HPV human papillomavirus, LBC liquid-based cytology preparation.
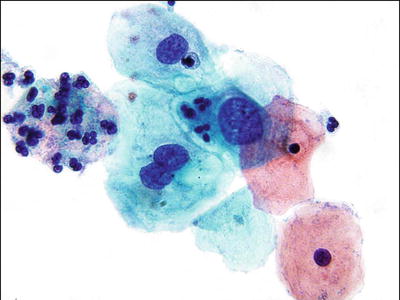
Fig. 1.31.
LSIL . Intermediate squamous cells with enlarged nuclei and hyperchromasia. LBC liquid-based cytology preparation.
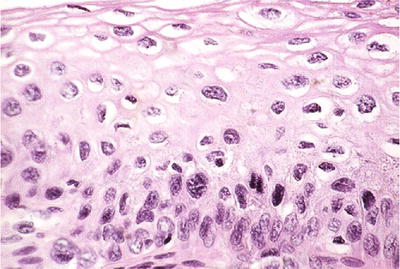
Fig. 1.32.
LSIL (CIN1). Disordered proliferation of dysplastic cells in the lower third of the epithelium and HPV effect toward the surface (histology, H&E).
Cytologic Features
♦
LSIL is characterized by nuclear enlargement greater than 3× size of an intermediate squamous cell nucleus (often 5–6× the size, greater than 150 μm2), hyperchromasia, and nuclear irregularities. The chromatin pattern is usually finely granular and uniformly distributed. Nucleoli are generally inconspicuous or absent
♦
HPV cytopathic effect is characterized by large perinuclear halos with sharp borders and dense surrounding peripheral cytoplasm (koilocytes). The nuclei of koilocytes are enlarged and hyperchromatic with slightly irregular nuclear contours
♦
Dyskeratosis, binucleation, multinucleation, and nuclear pleomorphism may be observed in these cells. Tumor diathesis is absent
♦
LSIL in LBC may demonstrate decreased hyperchromasia, increased nuclear detail, and more apparent nuclear membrane irregularities compared to CP smears
Differential Diagnosis
♦
HSIL exhibits significant increase in the N:C ratio, hyperchromasia, and marked chromatin irregularities. The cells are usually arranged in syncytial aggregates
♦
ASC-US cells tend to be few in number and have slight nuclear enlargement (2.5–3× intermediate cell nucleus). The chromatin pattern in ASC-US is finer and the nuclear membranes are less irregular
♦
LSIL cannot exclude HSIL (LSIL-H): Occasionally, a specimen is encountered with cytologic features that lie between LSIL and HSIL, especially if there are few cells that meet criteria of ASC-H that are identified in a background of LSIL. The 2014 Bethesda system nomenclature maintains that classification into either LSIL or HSIL is possible in most instances. The Bethesda system does not advocate the use of LSIL-H category, which does not have an associated ASCCP management guideline. There will remain, however, an occasional case in which it is not possible to categorize an SIL and a comment explaining the nature of the uncertainty may be employed. An interpretation of ASC-H may be made in addition to a LSIL interpretation, which should lead to colposcopic evaluation, especially in young women. This dual interpretation should comprise only a minority of cases
High-Grade Squamous Intraepithelial Lesion (HSIL) (Figs. 1.33, 1.34, 1.35, 1.36, and 1.37)
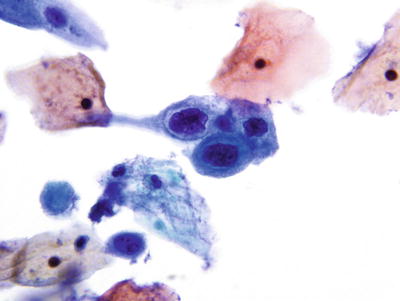
Fig. 1.33.
HSIL . Metaplastic cells with enlarged nuclei, irregular nuclear contours, and coarse chromatin pattern. Subsequent biopsy revealed HSIL (CIN2). LBC liquid-based cytology preparation.
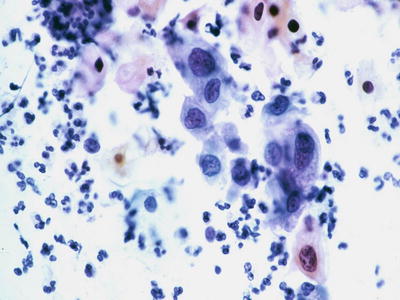
Fig. 1.34.
HSIL . Loose arrangement of small immature cells with high nuclear to cytoplasmic ratio, markedly irregular nuclear contours, and coarsely granular chromatic pattern (subsequent biopsy revealed HSIL/CIN2). CP conventional preparation.
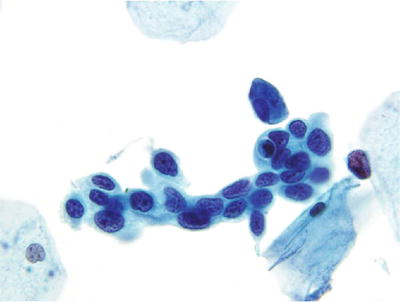
Fig. 1.35.
HSIL . Syncytial cluster of small cells with very high nuclear to cytoplasmic ratio, markedly irregular nuclear contours, and coarse but equally distributed chromatic pattern. LBC liquid-based cytology preparation.
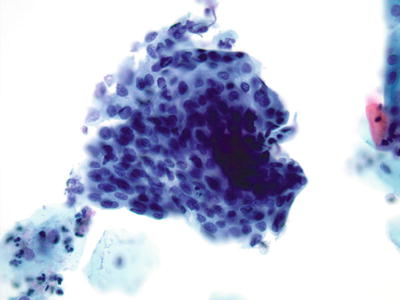
Fig. 1.36.
HSIL . Syncytial cluster of cells with enlarged nuclei, irregularities of nuclear contour, and coarse chromatin pattern. (Subsequent biopsy revealed HSIL/CIN3). LBC liquid-based cytology preparation.
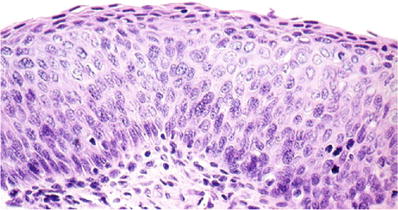
Fig. 1.37.
CIN3. Severe dysplasia (carcinoma in situ). Full-thickness disordered maturation with dysplastic cells showing hyperchromatic nuclei and high nuclear to cytoplasmic ratio (histology, H&E).
♦
The focus of cervical cancer screening is primarily aimed at detection and treatment of HSIL
Cytologic Features
♦
HSIL shows increased N:C ratio (the overall cell size and nuclear size, however, are smaller than LSIL) and abnormal nuclear features. In LBC, the nuclear membrane irregularities are distinctly prominent, and hyperchromasia is often observed as coarsening of chromatin material rather than darkening of the nuclear stain in CP smears. Generally, HSIL cells tend to be round to oval with lacy, delicate, or metaplastic cytoplasm
♦
HSIL often occurs in cell aggregates (hyperchromatic crowded groups) and syncytial-like arrangements. In LBC, HSIL cells may be fewer in number and more isolated or present in small groups due to processing. Tumor diathesis is absent
♦
“ Keratinizing dysplasia” refers to the pleomorphic appearance of the cells rather than cytoplasmic staining. The cells exhibit pleomorphism with marked anisonucleosis (caudate, spindle, elongated, tadpole, or bizarre forms) and dense orangophilic cytoplasm with distinct borders. It can be difficult to grade keratinizing dysplasia because it may show a greater degree of pleomorphism
Differential Diagnosis
♦
Other causes of hyperchromatic crowded groups can be confused with HSIL
♦
Invasive SCC is characterized by marked cellular pleomorphism, prominent nucleoli, and the presence of tumor diathesis
♦
Endocervical adenocarcinoma in situ (AIS) is characterized by a picket-fence arrangement of columnar cells with peripheral palisading (“feathering”) and granular cytoplasm. The nuclei are usually oval and monotonous. This contrasts with the disordered syncytial arrangement of HSIL cells, which are smaller and exhibit variation in nuclear size and shape. HSIL is much more common than AIS and in difficult cases an interpretation of HSIL is generally rendered. A note can be added that the possibility of glandular origin cannot be excluded
♦
It may be also difficult to distinguish keratinizing dysplasia from keratinizing squamous cell carcinoma (KSCC) . KSCC generally has less cohesive cells and more marked nuclear pleomorphism. As such, keratinizing dysplasia is sometimes designated as SIL-ungraded to assure that a biopsy is obtained to evaluate for the presence of invasive carcinoma
Cervical Squamous Cell Carcinoma (SCC ) (Figs. 1.38, 1.39, 1.40, and 1.41)
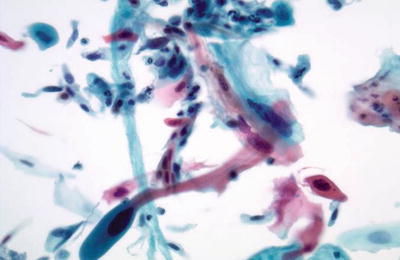
Fig. 1.38.
Squamous cell carcinoma (keratinizing type). Markedly pleomorphic orangophilic cells are present. The nuclei are irregular with enlarged hyperchromatic nuclei and irregular coarse chromatin pattern. LBC liquid-based cytology preparation.
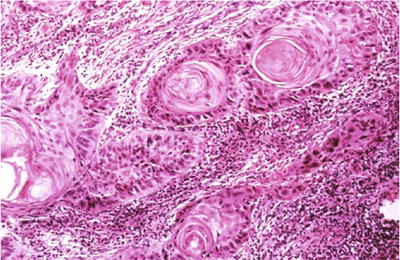
Fig. 1.39.
Invasive squamous cell carcinoma , keratinizing type. Keratin pearls are present in this invasive moderately differentiated squamous cell carcinoma (histology, H&E).
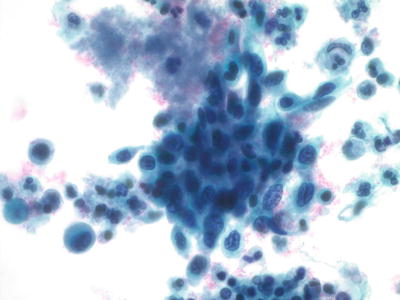
Fig. 1.40.
Squamous cell carcinoma (large cell nonkeratinizing type). Cluster of malignant cells with pleomorphic nuclei, irregular chromatin pattern, and nucleoli. LBC liquid-based cytology preparation.
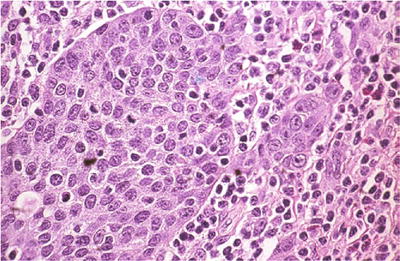
Fig. 1.41.
Squamous cell carcinoma, large cell nonkeratinizing type. Invasive squamous cell carcinoma that lacks squamous pearls (histology, H&E).
Cytologic Features
♦
SCC is characterized by single abnormal cells with loss of cellular and nuclear polarity. The carcinoma cells are about 1/5 or less the size of normal superficial or intermediate squamous cells. The nuclear size is about 2–4× that of intermediate squamous cell nucleus. The N:C ratio is very high and the chromatin is coarse and irregularly distributed with parachromatic clearing and nuclear molding
♦
Tumor diathesis, which consists of the host response of lysed blood, cellular debris, inflammatory cells, and protein precipitate, is generally observed in over 50% of cases
♦
In LBC, SCC cells may demonstrate a greater depth of focus of cell groups. The nuclei maintain features indicative of malignancy but are usually less hyperchromatic when compared to CP smears. In addition, in LBC, nucleoli are more prominent and there is a distinctive necrotic background pattern consisting of debris immediately surrounding individual cells or clusters of cells (clinging diathesis)
Variants
♦
Keratinizing SCC (KSCC) shows significant pleomorphism with spindle, bizarre, tadpole, and caudate cells. They have a dense keratinizing orangophilic cytoplasm with distinct cell borders. Their nuclei are pleomorphic and hyperchromatic with coarsely clumped chromatin and inconspicuous nucleoli. Anucleated squamous cells and atypical parakeratotic cells may be present in the background. KSCC may lack tumor diathesis (Figs. 1.38 and 1.39)
♦
Large cell nonkeratinizing (LCNK) SCC shows less anisocytosis and anisonucleosis. There are numerous abnormal single cells and syncytial groups of cells with high N:C ratio, dense cytoplasm, and indistinct borders. The nuclei tend to be round to oval with nuclear overlapping, coarse granular chromatin with chromatin clearing, and macronucleoli. Tumor diathesis is present (Figs. 1.40 and 1.41)
Differential Diagnosis
♦
HSIL cells do not exhibit significant nuclear molding, chromatin clearing, or tumor diathesis
Hyperchromatic Crowded Groups (HCG )
♦
Generally refers to readily identified three-dimensional aggregates of crowded cells with dark nuclei. Some may be reminiscent of microbiopsies
♦
The majority of cases are due to a benign or reactive process:
Examples: follicular cervicitis, exodus, atrophy, aggressively sampled endocervical cells, lower uterine segment endometrium, and benign mimickers of endocervical glandular atypia
♦
Less frequently (less than 5%) HCGs may represent sampling of a significant lesion, either squamous (discussed above) or glandular (discussed below):
Examples: HSIL, AIS, and adenocarcinomas of the endocervix or endometrium
♦
In general, HCGs can usually be recognized as to their respective nature, however, some remain difficult to interpret. All require careful review for features that favor a neoplastic process such as disorganized architecture, pleomorphic cells with anisonucleosis, atypical isolated cells, prominent nucleoli, and mitosis
Normal and Reactive Endocervical Cytology
Cytologic Features
♦
Normal endocervical cells may occur singly or in strips, rosettes, or sheets. They are usually elongated and columnar. When viewed on end, they are polygonal or cuboidal and demonstrate the typical “honeycomb” arrangement. The nuclei are round to oval and are often indented or possess a protrusion of the nuclear contents at one end. The nuclear chromatin is finely granular and evenly dispersed. Multiple small chromocenters and one or more small eosinophilic nucleoli may be present. Binucleation or multinucleation is not uncommon. The cytoplasm of endocervical cells is usually described as granular, but it may show fine vacuolization. Variability is observed in cells derived from various regions of the endocervical canal
♦
Endocervical cells with reactive/reparative changes occur in sheets and strips with minor degrees of nuclear overlap. They may exhibit nuclear enlargement, up to 3–5× the size of normal endocervical nuclei. Mild variation in nuclear size and shape occurs. Slight hyperchromasia is frequently evident. Nucleoli are often present. The cells usually have abundant cytoplasm. Distinct cell borders are discernible. Rarely mitotic figures may be seen in repair
Differential Diagnosis
♦
Neoplastic endocervical cells demonstrate crowding, secondary gland formation, and elongated or irregular nuclei. In addition, mitotic figures and apoptosis are often observed
Tubal Metaplasia (Figs. 1.42 and 1.43)
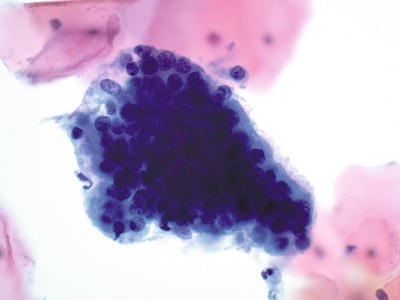
Fig. 1.42.
Negative for intraepithelial lesion or malignancy. Endocervical cells, tubal metaplasia. A strip of ciliated columnar endocervical cells. LBC liquid-based cytology preparation.
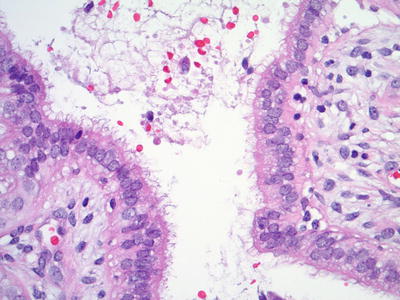
Fig. 1.43.
Tubal metaplasia . Ciliated tubal epithelium lines an endocervical gland (histology, H&E).
♦
A normal finding especially in the upper endocervical canal
Cytologic Features
♦
The presence of terminal bars and cilia is the characteristic feature. Cells tend to form small crowded cellular strips, clusters, or flat honeycomb sheets. They have evenly spaced, uniform, and basally oriented round to oval nuclei. The nuclei are finely granular with evenly distributed dark or washed-out chromatin. Small inconspicuous nucleoli may be observed. Mucus depletion and ciliocytophthoria may be present
Differential Diagnosis
♦
AIS cells lack terminal bars or cilia, show nuclear hyperchromasia and a coarse chromatin pattern, and demonstrate nuclear feathering and rosettes. Apoptosis is only observed in AIS
Microglandular Endocervical Hyperplasia
♦
Related to progesterone effect and may be related to pregnancy, birth control pills, and estrogen use in postmenopausal women
Cytologic Features
♦
Not distinctly recognized on cytology. Usually indistinguishable from endocervical reactive hyperplasia and regeneration/repair
♦
Large sheets of polygonal or columnar cells with minimal stratification, pseudoparakeratotic degenerative changes, and abundant fine or vacuolated cytoplasm. The nuclei are normal or mildly increased in size with normal or slightly hyperchromatic chromatin. Nucleoli may be present and prominent. Terminal bars and cilia may be present
Arias-Stella Reaction
♦
Nonneoplastic hormone-related phenomenon that is usually observed in pregnancy. Clinical correlation is essential for diagnosis
Cytologic Features
♦
The cytologic features are not specific and the interpretation is often that of atypical endocervical cells (AEC)
♦
Single or small groups of “atypical” glandular cells with low N:C ratio and abundant clear or faintly eosinophilic cytoplasm. Nuclear enlargement, hyperchromasia, pleomorphism, and nuclear grooves can be observed. Nuclei are often smudged with ground-glass appearance
Benign Glandular Cells in the Specimens from Post-Hysterectomy Women
♦
Prior to documenting the presence of benign glandular cells in the setting of a post-hysterectomy “vaginal Pap test,” it is important to assure that the “hysterectomy” was not supracervical. Also observe that the cells in question are truly glandular and not a mimic, such as atrophy
♦
The likely sources of benign glandular cells include prolapse of uterine tube, vaginal endometriosis, fistula, vaginal adenosis not associated with DES exposure, or glandular metaplasia associated with prior radiation or chemotherapy. Sometimes the reason remains unknown
♦
These findings are considered benign and do not warrant an interpretation/diagnosis of “atypical glandular cells” (AGC)
Glandular Cell Abnormalities
♦
The Pap test was not designed to screen for cervical glandular lesions and these abnormalities are more difficult to detect and interpret than squamous lesions. The improvement in sampling devices and the documented increase in endocervical adenocarcinoma have resulted in recognition of a spectrum of glandular changes, lesions, and mimics
♦
Whenever possible, atypical glandular cells (AGC) should be qualified as favor endocervical or favor endometrial in origin. When the distinction cannot be made, the cytology report of AGC should indicate the uncertainty as to the source of these cells
♦
The diagnosis of AGC in the typical cytology laboratory is infrequent (0.3–1%)
♦
For AGC and AEC, colposcopy with endocervical sampling is indicated. Cases diagnosed as AGC have approximately a 13% risk of CIN2 or worse and 2.8% risk of cancer. Endometrial sampling is added if the patient’s age is over 35 or they are having abnormal bleeding, regardless of HPV result. AEM is managed by endometrial and endocervical sampling
Atypical Endocervical Cells (AEC ) (Fig. 1.44)
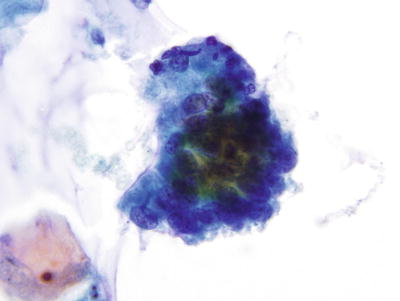
Fig. 1.44.
Atypical endocervical cells . A strip of pseudostratified atypical endocervical cells. LBC liquid-based cytology preparation.
♦
Cytomorphologic changes beyond those encountered in reactive processes, but qualitatively or quantitatively fall short of an unqualified diagnosis of AIS or endocervical adenocarcinoma
♦
Follow-up reveals that 20–40% of cases diagnosed as AEC are normal, reactive, or benign processes; 35–80% are derived from SIL; and 0–11% represent endocervical abnormalities. This demonstrates the inherent difficulties in the interpretation of these lesions. SIL may involve endocervical glands and mimic glandular cells
Cytologic Features
♦
Columnar cells with mild cellular crowding without nuclear pseudostratification, nuclear enlargement, hyperchromasia, and anisocytosis
Differential Diagnosis
♦
HSIL often demonstrates syncytial groups, opaque hyperchromatic nuclei, dense cytoplasm, and the presence of individual SIL cells
Atypical Endocervical Cells, Favor Neoplastic (Fig. 1.45)
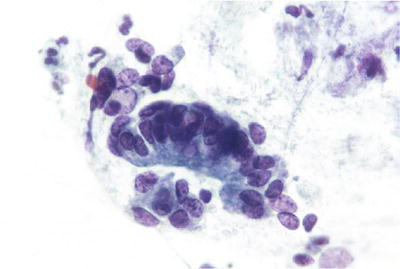
Fig. 1.45.
Atypical endocervical cells, favor neoplastic. A strip of pseudostratified atypical endocervical cells and a disorganized cluster of similar cells. The findings fall short of those characteristic of AIS. LBC liquid-based cytology preparation.
♦
Cells have some but not all the criteria as outlined for AIS below or other “atypical” presentation which should be individually and specifically categorized in a qualifying statement
Endocervical Adenocarcinoma In Situ (AIS ) (Figs. 1.46, 1.47, and 1.48)
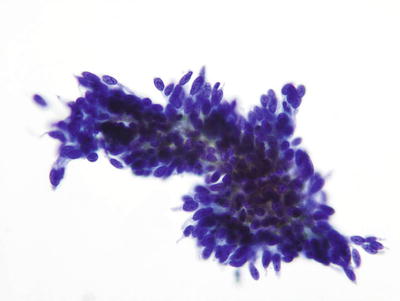
Fig. 1.46.
AIS. Hyperchromatic endocervical cells with pseudostratification and feathering. LBC liquid-based cytology preparation.
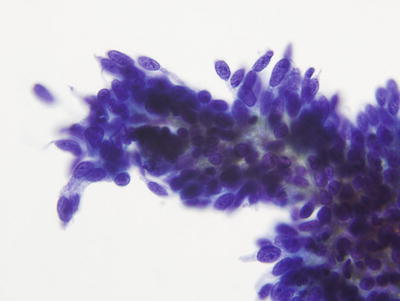
Fig. 1.47.
AIS. High power image of three-dimensional clusters of endocervical cells with enlarged elongated nuclei and coarsely granular chromatin. LBC liquid-based cytology preparation.
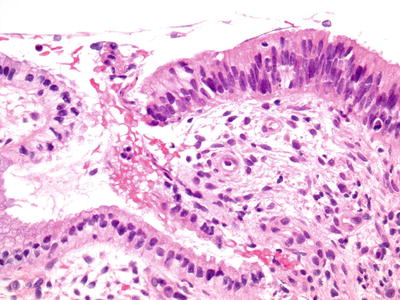
Fig. 1.48.
AIS. Endocervical gland with pseudostratification of enlarged hyperchromatic elongated nuclei adjacent to normal endocervical glandular epithelium (histology, H&E).
♦
Preservation of normal glandular architecture with involvement of part or all of the epithelium lining glands or the surface
♦
Associated with HPV 16 and 18 in 90% of cases
♦
Approximately 30–50% of AIS cases are associated with SIL
Cytologic Features
♦
“Endocervical type” of AIS is characterized by moderate to high cellularity of tight clusters and cohesive sheets of glandular cells showing pseudostratification, rosette formation, palisades, secondary gland openings, and feathering at the edge of sheets. The abnormal endocervical cells have uniform nuclear enlargement, hyperchromasia, nuclear irregularities, and crowding with elongated nuclei
♦
In LBC, AIS may manifest as hyperchromatic crowded groups (see above). These cells have dense immature cytoplasm, increased N:C ratio, large nuclei (70 μm2), nuclear overlap, even chromatin with coarse granularity, micronucleoli, mitosis, and apoptotic bodies. There is often difficulty with visualization of individual nuclei in the groups; a “disordered honeycomb” arrangement suggests an endocervical origin. Some architectural features may be more subtle in LBC, for example, the margins of the groups become smoother and sharper with lesser degrees of nuclear protrusion (feathering), but pseudostratified should still be evident
♦
The cytoplasm is delicate and finely vacuolated or granular with diminished mucin production
♦
The background is usually clean or inflammatory
Differential Diagnosis
♦
Tubal metaplasia is characterized by the presence of scant cellularity and cells with terminal bars and cilia. The nuclei are round to oval and more evenly spaced than those observed in AIS and the chromatin is finely granular. Mitoses, apoptosis, and nuclear hyperchromasia are not observed
♦
Repair/reactive cells are characterized by the presence of flat sheets of polygonal cells in a distinct honeycomb arrangement. Single atypical cells are absent. The cells have abundant cytoplasm and well-delineated cell borders. They are uniform cells with low N:C ratio. Minor degrees of nuclear overlap, nuclear enlargement, and variation in nuclear size and shape may be seen. The chromatin remains fine in reactive/reparative processes, but nucleoli can be prominent
♦
Microglandular hyperplasia is not distinctly recognized on cytology. Most cells look like normal endocervical cells or may assume repair-like features
♦
Cells derived from lower uterine segment endometrium typically occur as cohesive aggregates (tissue fragments) of a biphasic mixture of glandular and stromal cells (Fig. 1.49). The cell aggregates are large with tubular branched glands that may fold and appear three-dimensional. Feathering and palisading are absent. Cell polarity is retained. The nuclei are smaller and more uniform than those of AIS and have finely granular chromatin
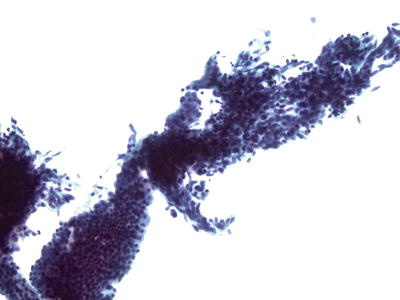

Fig. 1.49.
Lower uterine segment endometrium can mimic AIS. Branching fragment of epithelial and stromal cells. LBC liquid-based cytology preparation.
♦
HSIL involving endocervical glands may show feathering; however, it is often restricted to one end of a crowded sheet. HSIL cells are usually arranged in large syncytial aggregates with ill-defined cell borders and loss of polarity. HSIL cells have dense cytoplasm and hyperchromatic nuclei with nuclear membrane irregularities. Strips, rosettes, or pseudostratified atypical columnar cells that are characteristic of AIS are not observed in HSIL. Unlike AIS, numerous normal endocervical cells and individual dysplastic squamous cells are typically present in the background in HSIL. Since AIS is much less frequent than HSIL (0.01% compared to 0.2–0.4%), the latter interpretation should statistically be more likely to represent the true cervical abnormality (Figs. 1.50 and 1.51)
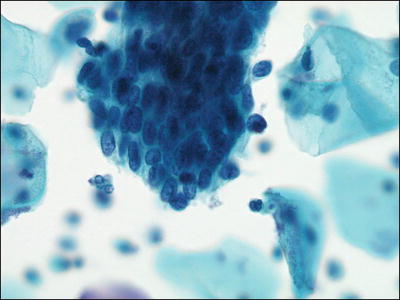
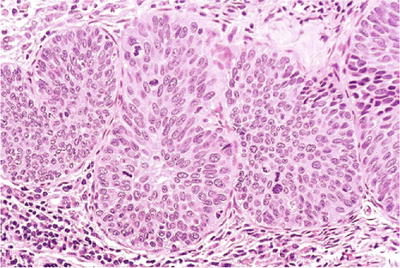

Fig. 1.50.
HSIL involving endocervical glands mimicking AIS. Syncytial cluster of HSIL in a tight cluster with rounded contour. LBC liquid-based cytology preparation.

Fig. 1.51.
CIN3; severe dysplasia/carcinoma in situ involving endocervical glands. The endocervical glands are occupied by dysplastic squamous cells extending from the surface (histology, H&E).
Endocervical Adenocarcinoma (Figs. 1.52 and 1.53)
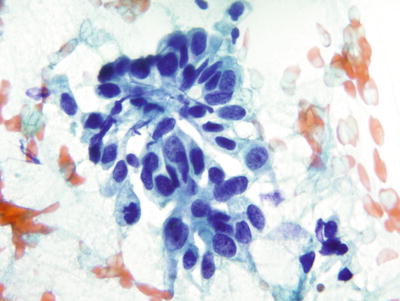
Fig. 1.52.
Adenocarcinoma , endocervical. Columnar cells with eccentric large round to oval nuclei, irregular chromatin, and nucleoli. CP conventional preparation.
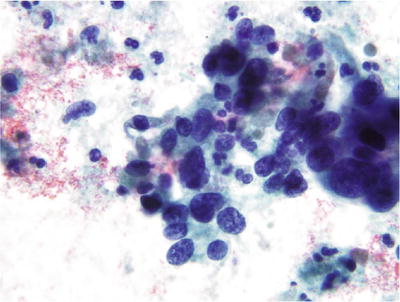
Fig. 1.53.
Adenocarcinoma, endocervical. Adenocarcinoma cells with suggestion of a columnar configuration and glandular foundation. The nuclei are elongated and hyperchromatic with irregularly distributed chromatin pattern and nucleoli. The cytoplasm is basophilic and granular. Tumor diathesis is present in the background. CP conventional preparation.
Cytologic Features
♦
Highly cellular specimens that demonstrate isolated single columnar or cuboidal cells and crowded three-dimensional or acinar groupings
♦
The nuclei are round to oval with coarse granular chromatin, irregular chromatin (clearing), and macronucleoli. An increase in nuclear size and nuclear pleomorphism is noted in less differentiated tumors. Multinucleation and mitotic figures may be present
♦
The cytoplasm is more abundant and foamy or finely vacuolated compared to AIS
♦
Necrotic granular (tumor) diathesis is present in approximately 50% of cases
Variants
♦
Minimal deviation adenocarcinoma (adenoma malignum) can be a very difficult cytologic diagnosis. In the proper clinical context, the presence of an excessively hypercellular specimen with strips, honeycomb sheets, and three-dimensional clusters of cells with distinct cell borders, abundant lacy cytoplasm, round to oval nuclei (rather than elongated) with coarse granular chromatin, and occasional nucleoli may suggest the possibility
Differential Diagnosis
♦
The features distinguishing endocervical from endometrial adenocarcinom a are shown in Table 1.2
Table 1.2.
Endocervical Adenocarcinoma Versus Endometrial Adenocarcinoma
Endocervical adenocarcinoma a (Endocervical type) | Endometrial adenocarcinoma (Endometrioid type) |
|---|---|
Younger patients | Elderly patients |
Sheets, strips, rosettes, feathering, and cell balls arrangement | Syncytial arrangement |
Larger columnar cells | Smaller cell with indistinct cell border |
Granular cytoplasm with mucin | Amphophilic cytoplasm without mucin |
Stroma absent | Stroma present |
Solitary macronucleoli | Multiple small nucleoli |
Necrotic dirty background | Watery diathesis |
Endometrial Cytology
Cytologically Normal Endometrial Cells (Figs. 1.54 and 1.55)
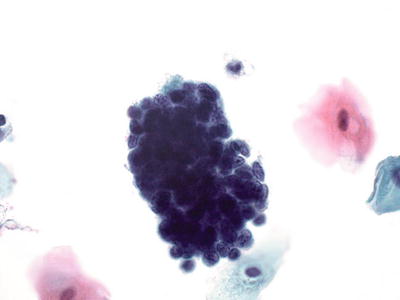
Fig. 1.54.
Endometrial cells, present in 45 years and older. Tight cluster of small glandular cells with eccentric nuclei and scant cytoplasm. The nuclei are uniformly round with regularly distributed chromatin. LBC liquid-based cytology preparation.
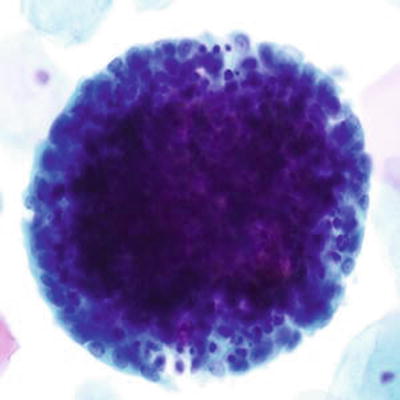
Fig. 1.55.
Endometrial cells, cytologically normal exodus. A tight ball of cells with central stromal cells and peripheral glandular cells. LBC liquid-based cytology preparation.
♦
Cervicovaginal cytology is a screening tool for SCC and its precursor lesions. It is not a sensitive or accurate screening test for detection of endometrial lesions and should not be used to evaluate suspected endometrial abnormalities
♦
The significance of endometrial cells is in part dependent on the age of the patient, day of last menstrual cycle, menopausal state, and hormonal usage:
Endometrial cells peak at days 4–5 and may persist until days 12–14 of a 28-day menstrual cycle
The presence of endometrial cells after days 12–14 or in postmenopausal women is associated with an age-dependent increased detection of endometrial pathology (endometrial polyp, hyperplasia, or adenocarcinoma)
♦
Changes/lesions that may be associated with endometrial cells include IUD use, hormonal effect, immediate postpartum period, impending or early abortion, acute and chronic endometritis, recent intrauterine instrumentation, endometriosis, polyps, and submucosal leiomyoma
♦
Abnormal shedding of cytologically normal endometrial cells may be associated with a 35–40% risk of endometrial pathology of any kind. There is advancing risk with age. Menopausal status may be omitted or inaccurately documented, especially in patients with irregular or excessive bleeding. The Bethesda system suggests reporting benign-appearing endometrial cells in all women age 45 and greater, regardless of hormonal therapy, to increase the positive predictive value and reduce unnecessary endometrial biopsies
♦
No endometrial sampling is usually done for asymptomatic, low-risk premenopausal women. Postmenopausal women with benign-appearing endometrial cells should have endometrial sampling
Cytologic Features
♦
The cytologic features of endometrial cells are related to their site of origin in the endometrium, menstrual cycle day, the environment into which they are shed, the interval that has elapsed since the cells were shed, the collection method, and the processing techniques:
Endometrial epithelial cells usually present as loose three-dimensional clusters and cell balls. The cells are small and cuboidal to round. The nuclei are round or bean shaped and slightly eccentric with uniform finely powdered chromatin pattern. The nuclear size is similar to that of an intermediate squamous cell nucleus. They have scant amphophilic and often finely vacuolated cytoplasm
Superficial endometrial stromal cells individually resemble, and may be indistinguishable from, histiocytes. They are identified as stromal cells when numerous and loosely clustered. They have round, oval, or reniform nuclei which are usually centrally located but may be eccentric. Their chromatin is finely granular and micronucleoli may be observed
Deep endometrial stromal cells present as loose aggregates late in the menstrual flow. The cells are spindle or oval with ill-defined cytoplasmic borders. The nuclei are reniform or cigar shaped, often with nuclear enfolding (grooves). The nuclear chromatin is finely granular but at times may be hyperchromatic. Small chromocenters may be observed
Exodus represents cell balls composed of central stromal cells surrounded by peripheral larger epithelial cells. Exodus clusters are usually observed on days 6–10 of the menstrual cycle
Atypical Endometrial Cells (AEM ) (Fig. 1.56)
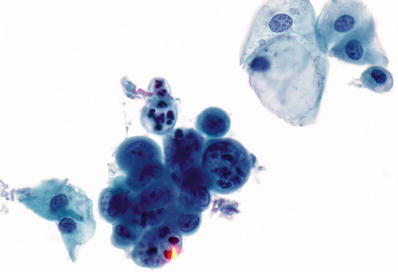
Fig. 1.56.
Atypical endometrial cells. Tight cluster of endometrial cells with loss of polarity, variation in nuclear size and shape, coarse chromatin pattern, and scattered small nucleoli. LBC liquid-based cytology preparation.
♦
There are no well-defined criteria to separate reactive versus preneoplastic endometrial cells, and atypical endometrial cells are not subdivided into categories
♦
May originate from endometrial polyps, acute and chronic endometritis, endometrial metaplasia, endometrial hyperplasia, or well-differentiated adenocarcinoma (FIGO grade 1). The incidence of adenocarcinoma in women with AEM is significantly higher in women greater than 59 years old
Cytologic Features
♦
Small groups composed of usually 5–10 cells with mild cellular crowding without nuclear pseudostratification. The nuclei are slightly enlarged with mild hyperchromasia, anisocytosis, and small nucleoli. The cell borders are ill defined, and when compared with endocervical cells, these cells have scant cytoplasm, which occasionally is vacuolated
Endometrial Adenocarcinoma (Figs. 1.57 and 1.58)
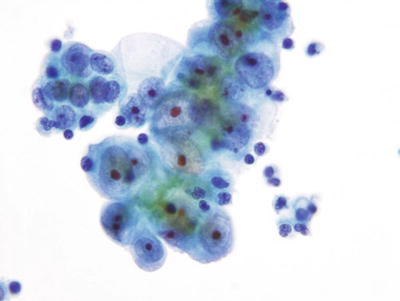
Fig. 1.57.
Adenocarcinoma, endometrial. Three-dimensional cluster of malignant glandular cells with round eccentric nuclei, vacuolated basophilic cytoplasm, and macronucleoli. LBC liquid-based cytology preparation.
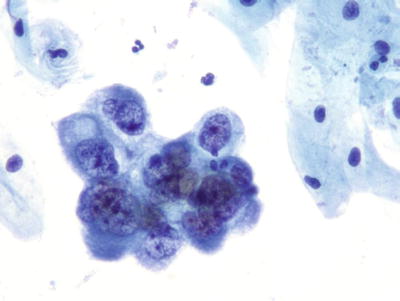
Fig. 1.58.
Adenocarcinoma, endometrial. A cluster of pleomorphic malignant glandular cells with eccentric irregular nuclei, irregular chromatin, and macronucleoli. LBC liquid-based cytology preparation.
Cytologic Features
♦
Shedding of neoplastic cells is generally sparse and irregular especially for low-grade tumors. The cell size increases from low- to high-grade tumors. In general, the cells present mainly as three-dimensional clusters of round cells and demonstrate loss of cell polarity
♦
The nuclei are enlarged (greater than 70 μm2) with high N:C ratio. Nuclear crowding and overlapping are typical. The nuclear chromatin is not usually hyperchromatic, but tends to be powdery with parachromatin clearing. Nucleolar enlargement is proportional to grade of tumor
♦
Cells tend to have a scant cyanophilic cytoplasm with fine vacuolization. The presence of intracytoplasmic neutrophils in glandular clusters may raise concern for at least AEM but only provided the associated endometrial cells are cytologically atypical and other clusters of endometrial cells in the Pap test are atypical
♦
In LBC, the endometrial adenocarcinoma cell groups may be more spherical or clustered than CP smears. The nuclei present in these groups may show less visual accessibility due to cellular overlap. The distinction between endometrial and endocervical adenocarcinoma may be more difficult in LBC than CP smears, especially for high-grade tumors
♦
Single or loose clusters of foamy histiocytes and lipophages may be present in the background as well as a watery granular tumor diathesis
Differential Diagnosis
♦
Normal endometrial cells have a round nucleus that is similar in size to the intermediate squamous cell nucleus. There are no cytologic features of malignancy
♦
The cytologic changes of AEM may overlap with those of low-grade endometrial adenocarcinoma. AEM cells maintain cell polarity, the nuclear size is less than that of adenocarcinoma, and there is no tumor diathesis
♦
The cytologic differences between endometrial and endocervical adenocarcinoma are shown in Table 1.2
Other Malignant Neoplasms
These tumors may represent rare or unusual variants of cervical carcinoma or uncommon primaries arising in the uterine corpus or adnexa that appear in the cervical cytology specimens either as exfoliated cells or through direct sampling of tumors
Small Cell Carcinoma of Cervix (Figs. 1.59 and 1.60)
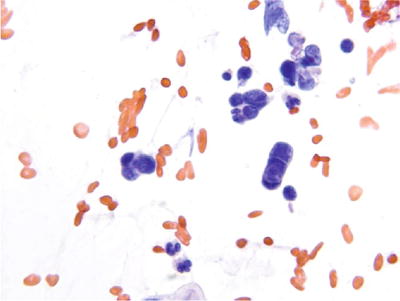
Fig. 1.59.
Small cell carcinoma . Malignant small cells with high nuclear: cytoplasmic ratio, molding, and single-file arrangement. LBC liquid-based cytology preparation.
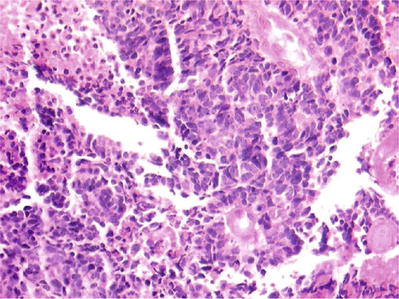
Fig. 1.60.
Small cell carcinoma. Small blue cells with molding and necrosis (histology, H&E).
♦
Uncommon and aggressive tumor of neuroendocrine origin. Almost all cases of small cell carcinoma are associated with HPV type 18 or 16. Small cell carcinoma of the cervix can coexist with adenocarcinoma or SCC
Cytologic Findings
♦
The neoplastic cells are generally small, cuboidal, or round and present as single cells or in small tight syncytial aggregates. The tumor cells have scant cyanophilic cytoplasm, a high N:C ratio, and round to oval nuclei with hyperchromatic and coarsely granular chromatin. Nucleoli are not observed. Nuclear molding, necrosis, and crush artifact are prominent
Papillary Serous Adenocarcinoma (Fig. 1.61)
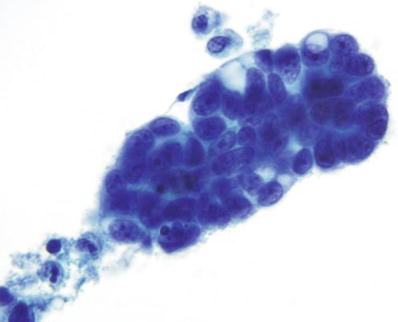
Fig. 1.61.
Vaginal specimen. Adenocarcinoma, metastatic to vagina from primary ovarian papillary serous carcinoma. Three-dimensional cluster of malignant glandular cells with prominent nucleoli. LBC liquid-based cytology preparation.
Cytologic Findings
♦
Cells are usually shed in papillary aggregates and have all the features of a poorly differentiated carcinoma. Small compact cell balls, elongated groups with peripheral molding, or irregular tight clusters may be identified. Occasionally the central connective tissue core may be seen. Psammoma bodies can be observed
Differential Diagnosis
♦
Papillary serous carcinoma of the endocervix, endometrium, and fallopian tubes/ovaries are indistinguishable. Poorly differentiated endometrioid adenocarcinoma (FIGO grade III) and extrauterine papillary serous carcinoma cannot be distinguished cytologically
Extrauterine Metastatic Adenocarcinoma
♦
The clinical history of a nongynecologic malignancy is essential for an interpretation of extrauterine carcinoma. Most extrauterine carcinomas are poorly differentiated adenocarcinomas
♦
Ovarian carcinoma is the most common primary site (papillary serous carcinomas). Other primary sites include the fallopian tubes, gastrointestinal tract, pancreas, and breast. The presence of ascites and patent fallopian tubes increases yield for extrauterine carcinoma
Cytologic Findings
♦
The specimens are generally of low cellularity if the carcinoma spreads via the fallopian tube and endometrium without implantation. The cellularity is high if there is implantation in the vagina or direct spread to the vagina
♦
May be three-dimensional, tubular, spherical, or papillary tissue fragments. The malignant cells are large cells with high N:C ratio, nuclear hyperchromasia, and macronucleoli
♦
Often no specific cytologic features suggest the primary site. Exceptions may include the presence of psammoma bodies (favor ovarian), columnar cells with brush borders (favor gastrointestinal), and cords of cells (favor breast)
♦
Tumor diathesis is generally absent in extrauterine carcinoma, provided implantation has not occurred
Malignant Mixed Müllerian Tumor (MMMT )
Cytologic Findings
♦
Characterized by the presence of two distinct tumor cell populations: a malignant poorly differentiated glandular component with or without squamous differentiation and a pleomorphic spindle or multinucleated sarcomatous component. Heterologous elements are rare and are cytologically difficult to recognize
♦
The malignant epithelial component can shed as single cells or in aggregates, whereas the sarcomatous cells usually occur as single cells and, rarely, in aggregates
♦
Tumor diathesis is usually evident
Lymphoma/Leukemia Cervix
Cytologic Findings
♦
Variable cytomorphology depending on the specific type of lymphoma
♦
There is complete lack of intercellular cohesion among tumor cells. Some of the cells may appear to cluster, but no true aggregates are present
♦
The tumor cell population is generally monomorphic and the lymphoma cells have high N:C ratio. In most lymphoma cells, the cytoplasm is barely visible; however, a minority has a plasmacytoid appearance with more abundant cytoplasm
♦
The nuclear membrane usually shows marked convolutions and may show a nipple-like protrusion. The chromatin is coarse with regular clumps. Nucleoli are not common except in the immunoblastic lymphoma category
♦
The cytologic subtyping of lymphomas is not reliable
Pap Test and HPV Testing
♦
Around 130 HPV subtypes have been described, of which 30 exclusively infect the anogenital area. HPV is divided into low risk and high risk according to their oncogenic potential:
High-risk HPV (HR-HPV) include types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66. Of the HR-HPV, HPV 16 is found in 50–60% of cervical cancers and HPV 18 in 10–20%. Types 26, 53, 68, 73, and 82 have also been detected in cervical cancer
The low-risk HPV types include 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and 89. Of the low-risk types, HPV 6 and 11 are the main causative agents for genital warts
♦
HPV “testing” refers to DNA testing for HR-HPV types. Studies have found no clinical role for low-risk HPV testing in cancer prevention. The management guidelines on the use of HPV testing are based on controlled trials using validated HPV assays. Similar results cannot be assumed when management is based on results of assays not similarly validated. Only HPV tests validated to ensure reproducibility and accuracy in identifying cancer precursors may be used by laboratories. The 2015 US Food and Drug Administration (FDA) Approved Screening Tests for HR-HPV include:
Hybrid Capture 2 (Qiagen, Gaithersburg, MD): in vitro nucleic acid hybridization assay with signal amplification and chemiluminescence for the qualitative detection of 13 types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) in cervical specimens. The ALTS studies employed ThinPrep® Pap test and Hybrid Capture 2 and serves as the basis for the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines
Cervista HR-HPV (Hologic, Bedford, MA): in vitro diagnostic test for the qualitative detection of DNA from 14 HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) in cervical specimens. Cannot determine the specific HPV type present. Uses Invader chemistry, a signal amplification method for detection of specific nucleic acid sequences. This method uses two types of isothermal reactions: a primary reaction that occurs on the targeted DNA sequence and a secondary reaction that produces a fluorescent signal
Cobas 4800 (Roche Molecular Systems, Pleasanton, CA): qualitative in vitro test utilizing amplification of target DNA by polymerase chain reaction (PCR) and nucleic acid hybridization for the detection of 14 HR-HPV types in a single analysis. The test specifically identifies types HPV 16 and 18 while concurrently detecting additional HR-HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The Addressing THE Need for Advanced HPV Diagnostics (ATHENA) trial serves as the basis for the approval of the Cobas 4800 HPV testing
APTIMA HPV and APTIMA HPV 16,18,45 Genotype (AHPV-GT) assay (Hologic, Bedford, MA): in vitro nucleic acid amplification test for the qualitative detection of E6/E7 viral messenger RNA (mRNA) from 14 HR-HPV (16, 18, 31.33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) in cervical specimens. The APTIMA HPV assay does not discriminate between the HR-HPV types. Testing can be performed on cervical specimens in ThinPrep Pap test vials containing PreservCyt Solution and collected with broom-type or cytobrush/spatula collection devices. The assay is used with the TIGRIS direct tube sampling (DTS) System. The Clinical Evaluation of APTIMA mRNA (CLEAR) study was the pivotal, prospective, multicenter clinical study in the United States to validate the APTIMA HPV assays
♦
The 2013 Cytology Education and Technology Consortium on HPV test utilization provided screening guidelines:
HPV testing is preferred for initial triage for: routine screening in women 30 years and older (as a component of co-testing), ASC-US in women 25 years and older, and LSIL in a women 30 years or older (as a component of co-testing)
HPV testing is also preferred for these preceding results for which co-testing surveillance (without colposcopy) is appropriate: women 30 years or older NILM/HPV positive, and women 25 years and older ASC-US/HPV negative, and women 30 years and older LSIL/HPV negative
The statement also includes recommendations as to when HPV testing is acceptable but not preferred: ASC-US in women 21–24 years and when the preceding results for which co-testing surveillance without colposcopy is ASC-US/HPV negative
In addition, guidelines for HPV testing are provided for preceding results for which co-testing surveillance is appropriate: postcolposcopic findings of CIN1 or less on biopsy and posttreatment testing surveillance for biopsy results of CIN2 or greater
Screening Guidelines
ASC, ASCCP, ASCP Screening Guidelines for Prevention and Early Detection of Cervical Cancer (Table 1.3)
Table 1.3.
Summary of Screening Guidelines
Age | Method | Management | Comments |
|---|---|---|---|
Less than 21 years | No screening. Regardless of age of sexual initiation or other risk factors | Cancer rates are extremely low. No HPV testing. Focus on vaccination | |
21–29 years | Screening with cytology alone every 3 years | ASCCP guidelines for ASC-US/HPV+ or LSIL or more severe | HPV testing should not be used for screening |
NILM or ASC-US/HPV−: Cytology rescreen in 3 years | |||
30–65 years | Co-testing every 5 years (preferred) | ASCCP guidelines for ASC-US/HPV+ or ≥LSIL | |
NILM/HPV+: 12-month follow-up with co-testing or test for HPV16 or HPV16/8 genotypes (HPV16 or HPV 16/8 +: colposcopy; if negative for CIN2, repeat co-test in 12 months. If HPV16 or HPV16/8−: 12-month follow-up with co-testing) | |||
Co-test negative or ASC-US/HPV−: Rescreen with co-testing in 5 years | |||
Cytology alone every 3 years (acceptable) | ASCCP guidelines for ASC-US/HPV− or ≥LSIL | ||
NILM or ASC-US/HPV−: Cytology rescreen in 3 years | |||
Older than 65 years | No screening if adequate prior screening NILM | If prior ≥CIN2: routine screening for at least 20 years. Screening is not resumed if there is a new sexual partner | |
Hysterectomy with cervix | No screening | Provided no history of ≥CIN2 in the past 20 years or cervical cancer at any time | |
HPV vaccinated | Recommended screening should not change (as unvaccinated/age category) |
HPV Prevalence and Management Guidelines
♦
HR-HPV is positive in 85% HSIL, 75–85% LSIL, 70–80% ASC-H, 50% ASC-US, and a variable percentage of normal Pap tests (10–20%)
♦
The probability of CIN2/3 is approximately 15% in ASC-US, 40–50% in ASC-H, 25–30% in LSIL, 50–90% in HSIL, and 30–50% in HSIL with negative colposcopic biopsy
♦
HPV “reflex” testing for equivocal ASC-US cytology provides effective triage for ASC-US and captures almost all HSIL cases (ALTS):
The negative predictive value for the HPV test is 99% (98.9–99.3%). The causes of false-negative results include few HSIL cells, low HPV DNA load (below cutoff for test), methodological or reproducibility errors, and histological interpretation errors. The absolute risk of greater than or equal to CIN3 with a negative HPV test, over a 10-year follow-up period, is very low (0.9%), compared to 6.9% with a positive HPV test
If the Pap test is negative and the HPV test is positive, then there is a 4% chance of having high-grade dysplasia within a 2-year follow-up period
The cumulative probability of HSIL (CIN2 or worse) within 2 years for ASC-US/HPV positive is 25–30%, ASC-US/HPV unknown is 15%, and ASC-US/HPV negative is 1–5%
ASC-US/HPV negative has a reported 3.0% and 1.4% absolute risk of greater than or equal to CIN2 or greater than or equal to CIN3 over a 2-year follow-up, respectively
The American Society for Colposcopy and Cervical Pathology (ASCCP) Guidelines (2012)
♦
The Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors are available at http://www.asccp.org. The concept of “equal management for equal risk,” along with balancing benefits and harms of screening, formed the basis of these management guidelines for abnormal cervical cancer screening tests and cancer precursors
♦
These guidelines should not be construed as directing an exclusive course of treatment. Some of the main guidelines for initial evaluation of abnormal Pap test are listed below
♦
Pap test-only strategies are limited to women younger than 30 years, but co-testing is expanded to women younger than 30 years in some circumstances
♦
Women aged 21–24 years are managed conservatively. Patients in this age group with ASC-US or LSIL should have repeat cytology at 1 year. If reflex HPV (acceptable for ASC-US only) was performed and is positive, repeat cytology at 1 year is also preferred. If the repeat cytology is ASC-H, AGC, or HSIL, then colposcopy is recommended. If the Pap test is negative, ASC-US, or LSIL, then a repeat Pap test in 1 year is recommended, at which time any finding of ASC or worse is referred to colposcopy
♦
ASC-US/HPV-negative patients should be followed with co-testing at 3 years. ASC-US/HPV-positive patients should be managed as patients with LSIL and undergo colposcopy
♦
Management of women over 30 with LSIL is dependent on the status of the HPV testing. Colposcopy is recommended for women with LSIL and no HPV test and in those with positive HPV test (as a result of co-testing). Subsequent management is dependent on whether CIN2–3 is identified or not identified, per ASCCP guidelines. For women with LSIL and negative HPV test, repeat co-testing at 1 year is preferred, but colposcopy is acceptable. At the 1-year repeat co-testing, an interpretation of ASC or above or a positive HPV test warrants colposcopy, while women with double-negative cytology and HPV test can be followed with repeat co-testing at 3 years. ASC-US/HPV-negative results are insufficient to allow exit from screening at age 65 years
♦
ASC-H and HSIL necessitate colposcopy and biopsy
♦
For pregnant women, the only indication for therapy is invasive cancer. Colposcopy is deferred in pregnant women at low risk for having cancer. Endocervical curettage is contraindicated. Management guidelines for pregnant women and other special populations with an abnormal Pap test interpretation are detailed in the ASCCP 2102 Consensus Guidelines of Management
♦
HPV testing is not recommended as a triage for glandular lesions. Colposcopy with endocervical sampling is recommended for all subcategories of AGC, AEC, and AIS. Endometrial sampling is added to colposcopy for AGC in patients over 35 years old or with irregular bleeding. For AGC that favor neoplasia, excision should be considered. Endometrial sampling is recommended, at any age, for an interpretation of AEM. Repeat cytology is not acceptable for AGC
♦
For women 30 years or older with negative cytology and positive HPV test (co-testing), repeat co-testing at 1 year is acceptable. Colposcopy is recommended if the 1-year repeat HPV test is positive or the cytology is ASC-US or worse. If both are negative, then repeat co-testing in 1 year is recommended. HPV genotyping is also acceptable; if HPV 16/18 genotyping is positive, then colposcopy is recommended. If both are negative, then repeat co-testing in 1 year is recommended
Primary HPV Testing
♦
Roche Cobas (Roche Molecular Systems, Inc., Pleasanton, CA) is the only test approved by the FDA for primary screening in United States
♦
Approved for ThinPrep® Pap test PreservCyt (Hologic, Marlborough, MA) cervical specimens collected using an endocervical brush and spatula
♦
In the proposed algorithm for primary HPV testing and reflex cytology, for women 25 years and older, if a woman has genotype 16/18, she is referred for colposcopy. If she is positive for any of the other 12 HR-HPV genotypes contained in the HPV assay, she has reflex cytology. If the Pap test is ASC-US, the woman is referred to colposcopy. Women negative on the initial HPV test continue routine triennial screening
♦
Potential limitations include that the internal beta-globin control is not specific for cervical epithelial cells and there is limited data on interfering substances. Additionally, cervical cancers with a negative HPV test have been documented
♦
HPV testing should only be performed in Clinical Laboratory Improvement Amendments (CLIA)-approved laboratories that perform proficiency testing and use HPV testing methods approved by the FDA with good laboratory quality assurance (QA) and internal validation
Quality Assurance (QA) Indicators
♦
The ALTS trial reported the benchmark for HPV-positive rate of ASC-US cases adjudicated by experienced pathologists to be 50.6%. In general practice the rate may at times be lower reflecting the patient population and the interobserver variability in daily practice. However, this benchmark and that provided by the CAP can provide valuable feedback to individual cytopathologists and the laboratory as a whole for improving the quality of gynecologic cytology interpretations
♦
Similarly, the ASC/SIL ratio, which can be calculated for the entire laboratory or for individual cytopathologists, can serve as a surrogate marker for the level of certainty and specificity. The Bethesda system proposed benchmark is that this ratio should not exceed 3:1. Later survey data by CAP found that the median ASC/SIL ratio in practice is 1.5 and the 95th percentiles are at 0.7 and 4.3 for ThinPrep Pap tests. A higher ratio suggests possible increased uncertainty and overuse of ASC interpretation. Over interpretation of both ASC and SIL can however keep this ratio within accepted guidelines
♦
It is important to note that neither the HPV-positive rate for ASC-US nor the ASC (ASC-US)/SIL ratio is a measure of diagnostic accuracy in gynecologic pathology. Established quality matrices are an essential component of each laboratory’s monitoring of performance and quality improvement
♦
Accuracy of gynecologic cytology can be measured by correlation with histopathologic follow-up. In general, most laboratories provide correlation for a negative biopsy with a preceding HSIL Pap test but some pursue cytologic-histologic correlation investigation of biopsy outcomes for ASC-US, ASC-H, and AGC as well
Cytologic and Histologic Correlation
♦
Sampling error is a major cause of noncorrelation. Even with specialized training and experience, colposcopic biopsy frequently misses the most significant pathology, including 66% of the CIN3
♦
The cytologic LSIL is not equivalent to histologic CIN1 (and cytologic HSIL is not equivalent to histologic CIN2/3). A biopsy diagnosis of CIN1 carries a lower risk than LSIL cytology or ASC-US/HPV positive because CIN1 implies that colposcopy biopsy has ruled out the most evident precancerous lesions
♦
There appears to be no difference in CIN3 capture with nurse practitioner, gynecologic oncology fellow, generalist, or gynecologic oncologist. The 2-year cumulative CIN3 or worse capture generally positively correlates with the number of biopsies taken
♦
Obtaining additional deeper levels can increase diagnostic accuracy when the precedent Pap test suggests significant abnormality and initial biopsy slides are negative. Similarly, deeper levels may assist in evaluation of an initially “negative” LEEP/cold-knife cone when the precedent biopsy is HSIL
♦
Immunohistochemical staining for p16, when performed on biopsies per the Lower Anogenital Squamous Terminology (LAST) guidelines, can support an interpretation of CIN2 and decrease interobserver variability. Similarly p16, along with selected deeper levels, can “salvage” initially “negative” LEEP/cold-knife cone, when the precedent biopsy is HSIL
Anal Cytology
Adequacy Criteria
♦
Satisfactory specimen is 2,000–3,000 well-visualized nucleated squamous cells for CP smears:
Roughly 1–2 nucleated squamous cells per high power field for ThinPrep®
Roughly 3–6 nucleated squamous cells per high power field for SurePath™
♦
External perianal skin is often keratinized, so anucleate squamous cells are common
♦
Unsatisfactory specimens may be due to excessive fecal material, poor preservation, or obscuring inflammation or bacteria
♦
Presence of rectal/colonic glandular cells or squamous metaplastic cells indicates transformation zone (anorectal junction) sampling; mention in report, but not necessary for adequacy
Anal Squamous Intraepithelial Neoplasia (SIL )/Anal Intraepithelial Neoplasia (AIN ) (Fig. 1.62)
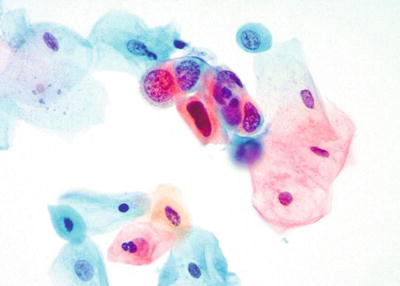
Fig. 1.62.
HSIL in an anal Pap test. High nuclear to cytoplasmic ratio, hyperchromasia, course nuclear chromatin, and irregular nuclear borders. LBC liquid-based cytology preparation.
♦
Incidence increasing in the United States and at-risk populations are likely under-screened
♦
Screening recommendations for men who have sex with men, women with a history of receptive anal intercourse or abnormal cervical Pap test results, and all human immunodeficiency virus (HIV)-infected persons with genital warts
♦
Other risk factors: transplant recipients and long-standing inflammation including fistulas, hemorrhoids, inflammatory bowel disease, or radiation
♦
Strongly associated with HR-HPV (most commonly HPV16)
♦
High-resolution anoscopy with biopsy of acetowhite epithelium is required after diagnosis of LSIL, HSIL, or ASC-H
Cytologic Features
♦
Use same terminology and cytomorphologic criteria as the Bethesda system for reporting cervical cytology
Atypical Squamous Cells of Undetermined Significance (ASC-US)
♦
Large nuclei greater than 2.5× the size of an intermediate squamous cell nuclei
Atypical Squamous Cells Cannot Exclude HSIL (ASC-H)
♦
Small cells with high N:C ratio that suggest HSIL, but fall short of all the criteria or are very low in number
Low-Grade Squamous Intraepithelial Lesion (LSIL)
♦
Large cells with nuclei greater than 3× the size of an intermediate squamous cell nuclei
♦
Koilocytes are somewhat less common in anal cytology
♦
Multinucleation is also less common
High-Grade Squamous Intraepithelial Lesion (HSIL)
♦
Small cells with high N:C ratio, hyperchromasia, and irregular nuclear borders
Ancillary Testing
♦
p16 demonstrates strong and diffuse staining in HSIL (AIN2, 3), HPV-ISH positive
♦
HPV DNA testing can be used with anal cytology to increase sensitivity, but not currently FDA approved
Differential Diagnosis
♦
Paget disease
♦
Melanoma
Anal Squamous Cell Carcinoma (SCC )
♦
Etiology, pathogenesis, and progression of anal squamous neoplasia analogous to cervical squamous neoplasia
♦
Majority are nonkeratinizing SCC arising in the anal canal. Keratinizing SCC is uncommon and arises from the perianal canal
Cytologic Features
Nonkeratinizing
♦
Small cells with increased N:C ratio, irregular nuclear borders, and coarse chromatin
♦
Diathesis may not be present
Keratinizing
♦
Pleomorphic cells with dense dark nuclei and orangophilic cytoplasm
Variants
♦
Verrucous carcinoma is well-differentiated
♦
Transitional zone SCC may show glandular or neuroendocrine differentiation
Differential Diagnosis
♦
Secondary involvement by metastatic SCC from the lung or direct extension from adjacent organs such as vulva in females
Paget Disease
♦
Intraepithelial tumor of apocrine gland origin
♦
15–50% of cases have an underlying malignancy
♦
Most often rectal adenocarcinoma
Cytologic Features
♦
Large pale to clear single cells with abundant cytoplasm difficult to diagnose on cytology
Immunohistochemistry
♦
Positive for mucicarmine, PAS, GCDFP, CK7, CEA, and EMA
Differential Diagnosis
♦
SIL/AIN: keratinocytes demonstrate intracellular bridges and rarely keratin formation
♦
Melanoma: positive for S100, HMB-45, Melan-A, and SOX10
Melanoma
♦
Rarely can be primary to the anal canal
♦
Highly aggressive
Cytologic Features
♦
Large single cells with pale to clear cytoplasm, enlarged nuclei, and prominent nucleoli. Can be cytologically diverse and may show spindled or plasmacytoid cells
Immunohistochemistry
♦
Positive for S100, HMB-45, Melan-A, and SOX10
Differential Diagnosis
♦
Paget disease
Organisms
♦
Special note: Neutrophils are abnormal in anal cytology. They are a clue to look for an infectious etiology
♦
Trichomonas
♦
Herpes
♦
Candida
♦
Cytomegalovirus
♦
Gastrointestinal amoeba
♦
Pinworm (Enterobius vermicularis) eggs
♦
Strongyloides
Part B: Nongynecologic Cytology
Overview
Overall Evaluation of Cytologic Smear
♦
Cellularity: high, moderate, or low
♦
Are the cells representative of a lesion?
♦
Type of the lesional cells (epithelial, mesenchymal, or lymphopoietic)
♦
Architectural arrangement of tissue fragments: clusters, sheets, papillary structures, etc
♦
Relationship of cells: honeycomb arrangement, crowded, overlapping, and disordered arrangement
♦
Cellular changes: nuclear to cytoplasmic (N:C) ratio, cytoplasm, and nuclear change (chromatin, nuclear membrane, and nucleoli)
♦
Background: bloody, granular, watery, proteinaceous, inflammatory, granuloma, mucoid, diathesis, etc
Key Cellular Features
Benign Cellular Features
♦
Centrally located, single or multiple, round to oval nuclei
♦
Chromatin finely granular and evenly distributed
♦
Nuclear membrane smooth
♦
Single or multiple, small, rounded nucleoli
♦
Normal N:C ratio
♦
Well-defined cytoplasmic border
♦
Tissue fragments are one- to two-cell layers with good polarity or honeycomb pattern
Reactive Cellular Features Due to Inflammation
♦
Centrally located, single or multiple, round to oval nuclei
♦
Nuclei enlarged with slightly enlarged nucleoli
♦
N:C ratio normal or slightly increased
♦
Chromatin slightly more granular and may be hyperchromatic
♦
Nuclear membrane may be wavy but uniform in thickness
♦
Background with inflammation and granular and cellular debris
Degenerative Changes Due to Reversible Injury
♦
Nuclear enlargement due to swelling (karyolysis)
♦
Chromatin smudged or washed-out
♦
Nuclear membrane may be uneven in thickness
♦
Nucleoli may be indistinct
♦
Cells may become more rounded due to swelling
♦
Cytoplasmic disintegration and fraying (moth-eaten appearance)
♦
Cell borders not well defined
♦
Normal N:C ratio
♦
Background may or may not be inflamed
Degenerative Features Due to Irreversible Injury
♦
Nucleus wrinkled and decreased in size
♦
Loss of chromatin detail
♦
Pyknotic and smudged chromatin
♦
Fragmentation and condensation of chromatin (karyorrhexis)
♦
Hyperchromasia
♦
Nucleolus invisible
♦
Cytoplasmic borders not well defined and disintegrated
♦
N:C ratio may be increased due to loss of cytoplasm or decreased due to nuclear shrinkage
Cellular Features of Repair (Fig. 1.63)
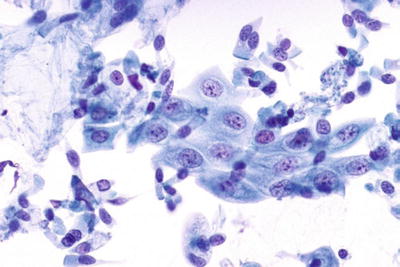
Fig. 1.63.
Repair, gastric brush. Monolayer sheet of well-organized cells with striking resemblance to each other. No cellular overlap. The nuclei are enlarged with prominent nucleoli and marginated, pale chromatin. The cell borders are well defined, and the cytoplasm shows streaming in the same direction. Papanicolaou stain, high power view.
♦
Monolayer sheets of well-organized cells and very few single cells
♦
Cells bear striking resemblance to each other
♦
Little or no cellular overlap
♦
Enlarged cells with centrally located round nuclei
♦
Enlarged nuclei
♦
Prominent nucleoli/cherry red single macronucleoli
♦
Marginated chromatin (swelling)
♦
Well-defined, uniform, and thick nuclear membrane
♦
Well-defined cell borders
♦
Cytoplasm may be streaming in the same direction (school of fish)
Atypical Repair
♦
Areas of atypical repair usually merge or coexist with typical repair
♦
Anisonucleosis within the monolayer sheet
♦
Increased cellular overlap within the sheet
♦
Increased number of single cells
♦
Increase chromatin granularity
♦
Altered nuclear polarity
♦
Hyperchromasia
♦
Cell borders blurred
General Features of Malignancy
♦
Increased N:C ratio (with few exceptions)
♦
Nuclei are round to oval, single or multiple, and frequently irregular
♦
Marked variation in nuclear size and shape (anisonucleosis)
♦
Chromatin is crisp and may vary from finely granular and evenly distributed to coarsely granular and unevenly distributed
♦
Chromatin rim may be irregular in thickness
♦
Nuclear membrane may be smooth but frequently irregular with grooves and sharp notches
♦
Nucleoli may vary from inconspicuous to prominent. They may be angulated or irregular
♦
Cellular discohesion with many single cells
♦
Cells within aggregates lose their polarity and form pseudosyncytia (cells blend with each other and lose their well-defined borders)
♦
Background may contain tumor diathesis which appears as granular material with cellular debris and old blood
Cellular Patterns
Mixture of Epithelial Cells/Lymphocytes
♦
Branchial cleft cyst
♦
Sialadenitis
♦
Lymphoepithelial lesion
♦
Warthin tumor
♦
Hashimoto thyroiditis
♦
Thymoma
♦
Seminoma
♦
Medullary carcinoma of the breast
♦
Nasopharyngeal lymphoepithelioma-like carcinoma
Mixture of Epithelial Cells/Spindle Cells (Biphasic)
♦
Pleomorphic adenoma
♦
Tumors with marked stromal fibrosis
♦
Neuroendocrine tumors (e.g., carcinoid)
♦
Synovial sarcoma
♦
Malignant schwannoma
♦
Mesothelioma (biphasic)
♦
Phyllodes tumor
♦
Brenner tumor
♦
Malignant mixed Müllerian tumor
♦
Leiomyoblastoma
♦
Wilms’ tumor
♦
Hepatoblastoma
♦
Epithelioid sarcoma
♦
Epithelioid leiomyosarcoma
♦
Melanoma
Plasmacytoid Cells
♦
Multiple myeloma
♦
Lymphoma (lymphoplasmacytic and Waldenström lymphoma)
♦
Carcinoid
♦
Medullary thyroid carcinoma
♦
Islet cell tumor
♦
Breast carcinoma, lobular and ductal
♦
Urothelial carcinoma
♦
Melanoma
Tumor with Discohesive Pattern
♦
Lymphoma
♦
Melanoma
♦
Sarcoma
♦
Squamous cell carcinoma
♦
Signet ring carcinoma
♦
Lobular carcinoma of the breast
♦
Seminoma
♦
Neuroendocrine tumors
♦
Small blue cell tumors: Ewing sarcoma, primitive neuroectodermal tumor (PNET), neuroblastoma, and rhabdomyosarcoma
Tumors with Acinar Pattern
♦
Prostate carcinoma
♦
Thyroid follicular tumor
♦
Carcinoid and neuroendocrine tumor
Tumor with Trabecular Pattern
♦
Hepatic tumor (hepatocellular carcinoma, hepatic adenoma)
♦
Thyroid follicular tumor
♦
Breast carcinoma
♦
Carcinoid
♦
Merkel cell carcinoma
Granuloma
♦
Infectious (e.g., fungal, mycobacterium, etc)
♦
Noninfectious (e.g., sarcoidosis, Wegener granulomatosis, etc.)
♦
Reaction to foreign body (e.g., keratin, amyloid, and suture material)
♦
Tumors with granulomatous component:
Squamous cell carcinoma (most common)
Other carcinomas with extracellular material, e.g., mucin
Seminoma
Hodgkin lymphoma
T-cell lymphoma
Intranuclear Cytoplasmic Invaginations/Pseudoinclusions
♦
Papillary thyroid carcinoma
♦
Medullary carcinoma of the thyroid
♦
Hürthle cell neoplasm
♦
Hyalinizing trabecular adenoma of the thyroid
♦
Parathyroid adenoma
♦
Bronchoalveolar carcinoma
♦
Hepatocellular carcinoma
♦
Melanoma
♦
Meningioma
♦
Sclerosing hemangioma
♦
Breast carcinoma
♦
Adrenal cortical carcinoma
Intracytoplasmic Inclusions/Hyaline Globules
♦
Papillary thyroid carcinoma (septate vacuoles)
♦
Bronchoalveolar carcinoma, clara cell type (surfactant)
♦
Squamous cell carcinoma (keratin)
♦
Adenocarcinoma (mucin droplets)
♦
Hepatocellular carcinoma (Mallory bodies or inspissated secretions)
♦
Melanoma
♦
Yolk sac tumor (alpha-fetoprotein)
♦
Pleomorphic liposarcoma (sarcoma bodies)
♦
Rhabdoid tumor (intermediate filaments)
Extracellular Hyaline Globules
♦
Corpora amylacea
♦
Collagenous spherules
♦
Liesegang rings
♦
Alveolar proteinosis
♦
Amyloid (irregular and thick)
♦
Mesothelial hyperplasia and mesothelioma (collagen balls)
♦
Clear cell carcinoma of the kidney or ovary
♦
Adenoid cystic carcinoma (smooth globules)
♦
Monomorphic adenoma (irregular globules)
Signet Ring Cells
♦
Goblet cells
♦
Mesothelial cells (degenerative change)
♦
Gastrointestinal tract carcinoma
♦
Mucinous carcinoma of the breast and ovary
♦
Mucin-producing carcinoid
Mucinous/Myxoid Background
♦
Mucinous carcinoma
♦
Pleomorphic adenoma
♦
Chondroid hamartoma
♦
Chondroid tumors
♦
Myxoid tumors
Psammoma Bodies
♦
Papillary carcinoma of the thyroid, breast, and ovary
♦
Bronchoalveolar carcinoma (BAC)
♦
Malakoplakia
♦
Meningioma
♦
Endosalpingiosis
♦
Mesothelial hyperplasia and mesothelioma
Respiratory Cytology
Overview
Normal Cellular Components of Lung Cytology
♦
Squamous cells: oral origin or due to squamous metaplasia
♦
Alveolar macrophages primarily dust cells
♦
Bronchial epithelial cells (ciliated columnar cells interspersed with goblet cells)
♦
Terminal bronchiolar and alveolar lining cells (pneumocytes)
♦
Inflammatory cells
♦
Megakaryocytes and mesothelial cells
Types of Pulmonary Specimens
♦
Exfoliative specimens include sputum, bronchial brushing (BB), bronchial washing (BW), and bronchoalveolar lavage (BAL)
♦
Fine-needle aspiration (FNA) include CAT scan-guided aspiration of pulmonary deep-seated masses and mediastinal sampling through endoscopically directed transbronchial and transesophageal aspiration
Spectrum of Cytologic Changes in Various Preparations
Sputum
♦
Best used to diagnose centrally located masses and has highest sensitivity for detecting SCC
♦
An adequate sample must contain alveolar macrophages (exception: acute pneumonia where severe acute inflammation predominates)
♦
May contain numerous benign squamous cells, Candida, Actinomyces, and food particles originating from the oropharynx. The presence of such elements in high numbers indicates saliva rather than deep cough sputum and renders it unsatisfactory for diagnosis
♦
A positive specimen often shows a small number of single or small tight clusters of tumor cells with frequent degenerative changes. Tumor diathesis is indistinguishable from inflammatory exudate or pneumonia
Bronchial Brushing and Washing
Clinical
♦
Useful in sampling mucosal and submucosal lesions that are directly visualized by the bronchoscope. They are also used to map the sites involved with tumor. Bronchial washing (BW) rather than bronchial brushing (BB) provides the best cost-efficiency particularly in well-visualized masses. Also useful in sampling peripheral lesions
Microscopic
♦
BB presents as large number of cells and tissue fragments with few macrophages. The predominant cells are columnar ciliated bronchial epithelial cells with or without reactive change. Profuse mucus can be present. Tumor diathesis if present is well demonstrated
♦
BW presents as large number of alveolar macrophages and scattered bronchial cells. Positive samples tend to contain small numbers of tumor cells
Bronchoalveolar Lavage
Clinical
♦
Samples the distal airway spaces and intraalveolar contents. Useful in the workup of lung infiltrate and opportunistic infections
Microscopic
♦
Consists predominantly of alveolar macrophages (pigmented, single, and multinucleated cells) and few ciliated cells. Inflammatory or infectious lesions will present with a considerable component of inflammatory cells depending on the disease
Image Guided Transthoracic (Percutaneous) Fine-Needle Aspiration of Lung Nodules
Clinical
♦
Fine-needle aspirations are useful in the workup of lung nodules particularly the peripheral ones. Can be more accurate in typing tumors and in distinguishing a benign, e.g., granuloma or hamartoma from a malignant mass
Microscopic
♦
A diagnostic sample will consist predominantly of the lesional cells admixed with few macrophages, bronchial epithelium, and rare mucus. Pneumocytes may be abundant with reactive changes
♦
Lesional cells could be aspirated in large numbers and may present with a pattern that reflects the original architecture of the lesion, e.g., papillae, single discohesive cells or large sheets, etc. Tumor diathesis is well demonstrated
♦
Well-prepared smears have excellent cellular preservation and good representation of lesion
♦
Mesothelial cells may be seen (picked up by the needle in its path)
Endoscopically Directed Bronchial and Mediastinal Sampling
♦
Transbronchial aspirates (TBNA) and transesophageal aspirates (TENA):
TBNA through rigid bronchoscope (1940)
TNBA through flexible bronchoscope (Wang needle 1970)
Improved peribronchial sampling
Restricted to low paratracheal (stations 4L and 4T) and subcarinal (station 7) lymph nodes (LN) that measure 2 cm or more
Performed blindly and requires estimation of location of lymph node by other imaging modalities
Does not allow real-time visualization or needle penetration into the area of interest
Needle trajectory may change and becomes parallel to the scope resulting in abundant bronchial sampling
♦
Endoscopic Ultrasound-Guided (EUS) FNA
Provides real-time imaging of mediastinal structures adjacent to major airways
Transesophageal EUS-FNA has limited access to LN stations 2L, 4L, 7L, 8L, and 9L. LN station 5 is not accessible
♦
Endobronchial ultrasound-guided FNA (EBUS)
More sensitive than transbronchial needle aspirate and can sample areas inaccessible by TBNA
Allows for sampling of nodal stations in the hilum and mediastinum
Rapid on-site evaluation (ROSE) increases the value of EBUS FNA for patients, as it allows for:
Assessment of specimen adequacy
Additional passes/needle redirection
Finalizing sampling when diagnostic material is obtained
Provides preliminary diagnosis in urgent cases
Specimen triage for ancillary studies: flow cytometry, microbiologic cultures, IHC, and molecular testing
Clinical
♦
Performed to sample a bronchial mass for primary diagnosis or mediastinal lymph nodes for staging and/or primary diagnosis and determining eligibility for surgery in non-small cell carcinomas (NSCC)
♦
Performed for surgical restaging of NSCC after radiation and chemotherapy
♦
Confirm clinically and radiologically suspected sarcoidosis by presence of nonnecrotizing granulomas
Microscopic
♦
A bronchial mass FNA will consist of the lesional cells admixed with bronchial cells, histiocytes, and mucin
♦
Aspirates of positive hilar lymph nodes will consist of the lesional cells admixed with bronchial cells and histiocytes. Lymphocytes may be present in the background in variable numbers depending on degree of replacement of the lymph node by the metastatic tumor
♦
Aspirate of a negative lymph node should contain lymphocytes to document adequate sampling of the node when the aspirate is negative
Benign Lesions
Pneumonia
Microscopic
♦
Specimens from bacterial pneumonia are cellular and consist predominantly of numerous polymorphonuclear leukocytes that may obscure other elements such as native respiratory cells. The background may contain cellular debris that mimics tumor diathesis and careful screening of smears with purulent material is warranted to rule out a necrotic tumor
♦
The presence of bacterial colonies is significant only in FNA samples and additional passes should be requested for cultures
♦
Specimens from fungal pneumonia particularly blastomycosis can contain severe acute inflammation, and smears need to be carefully screened to detect the spores
♦
Specimens from viral pneumonia will have high lymphocytic background
♦
Smears representing either viral or fungal pneumonia may contain scattered highly atypical squamous cells
♦
Other cellular changes such as reactive, e.g., creola, bodies or repair and degenerative features may be present
Differential Diagnosis
♦
Unsatisfactory exfoliative specimen due to oropharyngeal contamination. These samples tend to contain other contaminants such as squamous cells, Actinomyces, and Candida
♦
KSCC, when atypical squamous cells are present. Carcinoma usually presents with higher numbers of malignant cells with well-preserved hyperchromatic nuclei while those in pneumonia tend to show evidence of degenerative changes. The presence of viral inclusions or fungal elements is a clue to the nature of the atypia
Creola Bodies (Bronchial Cell Hyperplasia) (Fig. 1.64)
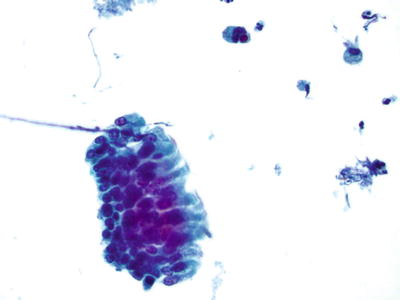
Fig. 1.64.
Creola body, bronchoalveolar lavage. Three-dimensional, tightly cohesive clusters bordered by cilia. Papanicolaou stain, high power view.
Clinical
♦
Originally described in asthmatic patients. The term “creola” was coined in honor of the patient whose sputum contained such clusters. Now it is established that such clusters can be seen in a variety of reactive conditions
Microscopic
♦
The smears can be very cellular and contain numerous tight clusters of benign reactive columnar ciliated cells Florid cases may contain papillary tissue fragments or three-dimensional cell balls
♦
These clusters are characterized by the presence of peripheral palisading cells with terminal plates and cilia sometimes intermixed with an occasional goblet cell. Central cells are smaller, less differentiated, and difficult to visualize clearly
Differential Diagnosis
♦
Adenocarcinoma
Clusters of highly atypical cells with nuclei situated at the periphery. There is usually evidence of cell discohesion, mitotic activity, and apoptotic figures. Bronchial hyperplasia, despite the cellularity, does not exhibit any of these malignant features. The presence of goblet cells and terminal cilia is clue to the benign nature of the cells
Pneumocyte Type II Hyperplasia
Clinical
♦
These cells are the result of regeneration after injury replacing the type I pneumocyte. Injury could have resulted from a variety of conditions including pneumonia, pulmonary infarction, diffuse alveolar damage (DAD), etc
Microscopic
♦
The number of cells may vary from few to numerous. The atypical pneumocytes may present as single cells, small clusters, or many large clusters. The latter is mainly encountered in cases with DAD
♦
The clusters are tightly cohesive with scalloping borders due to protruding nuclei at the periphery. The cells may have slightly enlarged nuclei but relatively normal or slightly increased N:C ratio
♦
The cells can be highly vacuolated and the vacuoles are frequently out of proportion with the cellular size
♦
Background may contain evidence of other reactive changes such as repair
Differential Diagnosis
♦
Bronchial cell hyperplasia
Clusters have focal areas with well-visualized terminal bars and cilia. Clusters may be in continuation with rows of reactive bronchial cells
♦
Adenocarcinoma
Present with more obvious atypia and evidence of discohesion. They tend to be finely vacuolated, rather than the large out of proportion vacuoles seen in pneumocyte hyperplasia
Pulmonary Infarct
Clinical
These are usually aspirates performed when a localized lesion is seen in a patient with a clinically undetected embolic event
Microscopic
♦
Smears are variable in cellularity and content depending on the area sampled. Sheets and papillary groups of reactive pneumocytes may be seen if the periphery of the lesion is sampled, while atypical squamous metaplastic cells are seen when the center is sampled
♦
Hemosiderin-laden macrophages may be present in the background and are a clue to the diagnosis
♦
Maximum atypia is seen during second to third postinfarction weeks
Differential Diagnosis
♦
Adenocarcinoma:
The cells are more pleomorphic and discohesive with obvious disorganized arrangement within the sheets or clusters
Cigarette and Habitual Marijuana Smoking
♦
The smears of exfoliative samples are generally cellular with features of irritation and consequent reactive changes
♦
The earliest change is reserve cell hyperplasia, which presents as tight clusters of uniform small cells with hyperchromatic nuclei This is followed by squamous metaplasia (Fig. 1.65)
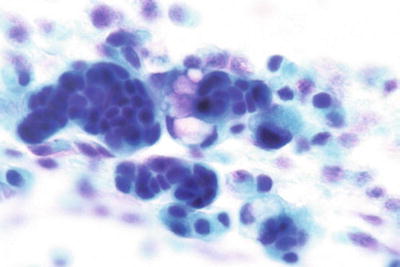

Fig. 1.65.
Reserve cell hyperplasia, bronchial wash. Tightly cohesive small blue cells with no cytologic atypia or discohesion. The presence of contiguous goblet cells is a clue of their origin. Papanicolaou stain, high power view.
♦
Background of numerous multinucleated histiocytes frequently containing anthracotic pigment
Radiation and Chemotherapy-Induced Atypia (Fig. 1.66)
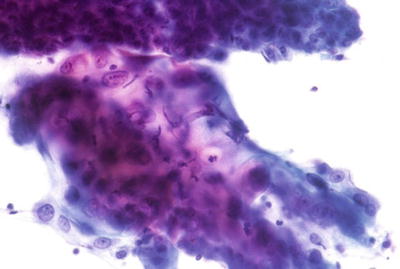
Fig. 1.66.
Radiation therapy change, lung, FNA. Cytomegalic cells arranged in a syncytium. The cells are bizarre with amphophilic, frequently vacuolated cytoplasm and ingested neutrophils. The chromatin is frequently smudged and difficult to read. Papanicolaou stain, high power view.
Clinical
♦
Patients undergoing treatment for a malignant disease
♦
Acute radiation therapy changes are seen within 6 months of initiating treatment, while chronic changes may persist for a long time. Chemotherapy changes resemble those of chronic radiation and subside within a month of terminating therapy
Microscopic
♦
Acute radiation therapy:
Cytomegaly, nucleomegaly, multinucleation, and normal N:C ratio
Cytoplasmic vacuolization and neutrophil ingestion (vacuoles may be prominent) and cytoplasmic polychromasia
Syncytial sheets with blurred cell borders. Cytoplasm may be very fragile and blend with the background
♦
Chronic radiation and chemotherapy changes
Cytomegaly, nucleomegaly, multinucleation, and normal N:C ratio
Syncytial sheets with blurred cell borders
Bizarre cells with amphophilic cytoplasm, indistinct cell border, and cytophagocytosis of neutrophils
Nuclear degeneration (karyorrhexis and karyolysis), hyperchromasia, and prominent nucleoli
Smudged chromatin and poor nuclear detail
Differential Diagnosis
♦
Poorly differentiated carcinoma:
These malignant cells will have increased N:C ratio. The nuclei are hyperchromatic and frequently contain prominent nucleoli. The chromatin is well preserved and nuclear detail is evident
Amiodarone-Induced Changes
Clinical
♦
Patients treated with amiodarone for supraventricular arrhythmia
♦
Patients present with nonspecific symptoms such as cough, shortness of breath, etc. and frequently have bilateral diffuse lung infiltrates
Microscopic
♦
Cellular smears consisting of numerous foamy alveolar macrophages with uniform, well-defined, and distended vacuoles. These cells are negative for oil red O stain
♦
Atypical pneumocytes with increased N:C ratio, hyperchromasia, and nuclear pleomorphism
♦
Background of increased lymphocytes, neutrophils, and eosinophils
Electron Microscopy
♦
Osmiophilic lamellar cytoplasmic inclusions
Differential Diagnosis
♦
Lipoid pneumonia (Fig. 1.67)
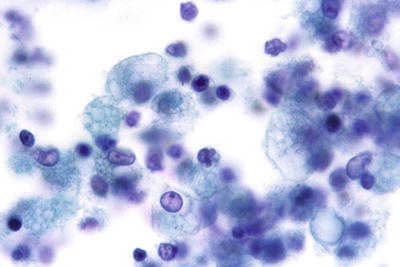

Fig. 1.67.
Lipoid pneumonia, bronchoalveolar lavage. Lipid-laden macrophages with highly vacuolated cytoplasm. Papanicolaou stain, high power view.
Patient presentation is different and lack history of amiodarone therapy (see below)
Vacuoles are less defined and positive for oil red O
Hemosiderin-Laden Macrophages
Clinical
♦
Pediatric patients
Idiopathic pulmonary hemosiderosis (isolated)
Pulmonary hemosiderosis associated with sensitivity to cow’s milk
Glomerulonephritis
Collagen vascular and pruritic disease
Cardiac diseases, intravascular lesions, or malformations
♦
Adult patients
Diffuse interstitial pulmonary disease
Congestive heart failure (heart failure cells)
Hypersensitivity pneumonitis
Fibrosis associated with rheumatoid arthritis
Radiation or chemotherapy
Microscopic
♦
Smears contain many pigmented histiocytes. Hemosiderin appears as refractile particles that vary in size and stains as golden brown with Papanicolaou and as greenish brown with Diff-Quik stain
Quantitation of Hemosiderin-Laden Macrophages
♦
Percentage of cells containing hemosiderin (Prussian blue or Perl stain)
Result of >20% has a sensitivity of 10% and specificity of 92% for detecting pulmonary hemorrhage and hemosiderosis
♦
Hemosiderin score
Adding staining intensity score (0–3) for each 100 cells measured
A score of >50 has a high sensitivity and specificity in the proper clinical setting
Differential Diagnosis
♦
Carbon-laden histiocytes (dust cells)
These cells are commonly seen in all samples and are particularly increased in smokers. They contain anthracotic pigment which is nonrefractile and shows minimal variation in size. The pigment stains dark brown or black on both Papanicolaou and Diff-Quik stains
♦
Melanophages
These cells are seen in patients with history of melanoma but are not always associated with the malignant cells. The pigment varies from fine dust like quality to globular droplets and stains brown to black
Lipid-Laden Macrophages in Bronchial Lavage (See Fig 1.67)
Clinical
♦
Associated with tracheal aspiration in children with gastroesophageal reflux (GER) or lobular consolidation distal to an obstructed bronchus. In adults it may also be associated with aspiration of mineral oil
Microscopic
♦
Smears with finely vacuolated histiocytes admixed with bronchial cells. Vacuoles are fine and ill defined
♦
Fresh specimens should be stained with oil red O to confirm the diagnosis
♦
Quantitation/percentage of oil red O-positive cells may be of value for predicting recurrent aspiration
Corpora Amylacea (Fig. 1.68)
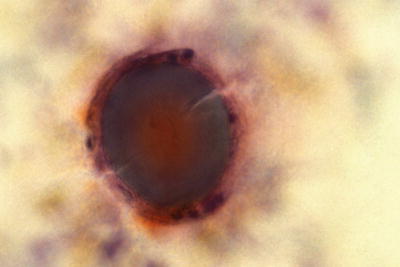
Fig. 1.68.
Corpora amylacea , bronchial wash. Thick amorphous material with internal rings. Note the crack induced during preparation and the surrounding giant cell. Papanicolaou stain, high power view.
Clinical
Their presence has no particular clinical significance and is seen in patients with:
♦
Older age
♦
Heart failure
♦
Pulmonary infarction
♦
Chronic bronchitis
Microscopic
♦
Variably sized round to oval dense bodies that stain amphophilic with Papanicolaou stain. They have concentric laminations and a radiating array arising from a central nidus. They frequently crack during smearing and can be seen phagocytosed by giant cells
♦
They are positive for periodic acid-Schiff (PAS) stain and resist digestion and are weakly positive with Congo red stain
Differential Diagnosis
♦
Alveolar proteinosis
Patients are symptomatic with dyspnea, dry cough, and fever. Radiologically, these patients have extensive bilateral consolidation. The material stains strongly positive with PAS and resists digestion and is also positive to oil red O stain but negative to mucin stains
♦
Amyloid
Patients may have systemic amyloidosis or history of lymphoma or carcinoid. Amyloid is rarely detected in exfoliative samples. It appears as flocculent irregularly shaped material that stains eosinophilic or cyanophilic with Papanicolaou stain and blue with Diff-Quik stain. Amyloid stains with Congo red and exhibits apple green birefringence upon polarization. It can be weakly reactive to PAS
Alveolar Proteinosis
Clinical
♦
This autoimmune disease is characterized by accumulation of phospholipoproteinaceous material within the alveolar spaces. Patients present with nonspecific symptoms such as cough or dyspnea, but radiologically they may present with bilateral extensive consolidation that is way out of proportion with their symptoms. In up to 50%, the patients may present with patchy and perihilar infiltrate “bat wing pattern”
Microscopic
♦
The lavage is usually thick and grossly opaque
♦
Microscopically it appears as amorphous thick material with irregular shapes that stains basophilic with Diff-Quik and amphophilic with Papanicolaou stain. The material is also strongly positive to PAS and oil red O (on fresh smears) but negative to all mucin stains and Congo red
Differential Diagnosis
♦
Corpora amylacea (see above)
♦
Amyloid (see above)
Sarcoidosis(Fig. 1.69)
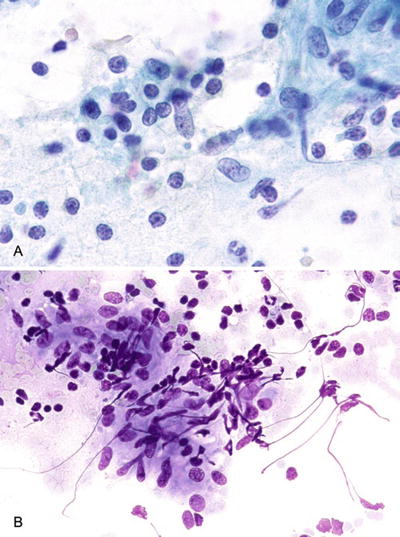
Fig. 1.69.
Sarcoidosis, FNA of lung. (A) Clusters of epithelioid histiocytes mixed with lymphocytes, Papanicolaou stain. (B) Diff-Quik stain, high power view.
Clinical
♦
This is a multiorgan disease that frequently affects the lung. Radiologically patients present with bilateral hilar and mediastinal lymphadenopathy and nodular lung infiltrate. The nodules are particularly numerous along the bronchi and vessels
Microscopic
♦
On FNA, may see loose clusters of epithelioid cells and giant cells intermingled with lymphocytes. These represent the noncaseating granulomata seen on histology
♦
BAL findings are not specific, but generally the number of lymphocytes and histiocytes is increased and many multinucleated giant cells can be detected. Schaumann bodies may be noted in the giant cells. Asteroid bodies are not specific. All microorganism stains are negative
Differential Diagnosis
♦
Other infectious diseases such as tuberculosis
Acid-fast bacilli may be detected by Ziehl–Neelsen stain in up to 40% of cases
♦
Other granulomatous diseases
Fungal infections: Granulomas are usually less cohesive and there may be evidence of necrosis and suppurative reaction especially with blastomycosis. Fungal spores and hyphae are detected and will stain with Gomori methenamine silver (GMS)
♦
Rheumatoid granulomas
Lung nodules are usually seen in severe cases and frequently associated with pleuritis and effusion. FNA will contain necrotic debris, scattered epithelioid histiocytes, and lymphocytes
Miscellaneous Findings
Ferruginous Bodies (Fig. 1.70)
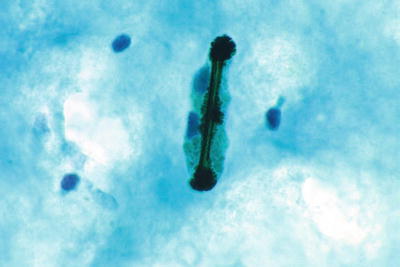
Fig. 1.70.
Asbestos body, bronchial wash. Fiber with a clear, colorless center, and uniform thickness. Papanicolaou stain, high power view.
Clinical
♦
General term applied to a variety of mineral fibers that have been inhaled and sheathed with golden brown iron–protein complex. Asbestos bodies are indicative of occupational asbestos exposure
Microscopic
♦
Consist of a clear colorless central fiber of uniform thickness within a golden brown iron–protein complex
Differential Diagnosis
♦
Non-asbestos bodies: have a black or yellowish center that is not uniform in thickness
Ciliocytophthoria
Clinical
♦
The presence of this finding is usually suggestive of viral infection (e.g., respiratory syncytial virus)
Microscopic
♦
Scattered anucleated cytoplasmic fragments bearing cilia are seen among the normal component of an exfoliative specimen such as bronchial cells and histiocytes. These fragments represent detached bronchial cilia
Asthma
Clinical
♦
The following findings are frequently encountered in asthmatic patients although they are by no means specific to asthma:
Curschmann spirals
•
Appear as coiled strands of inspissated mucus frequently associated with bronchial cell hyperplasia
Charcot-Leyden crystals
•
Elongated red crystalline structures derived from eosinophil phospholipase B, involved in prostaglandin metabolism
Bronchial cell hyperplasia
•
Also known as “creola bodies” (see description above). This entity was first described as a characteristic of status asthmaticus, but it is believed now that it is rather a feature of chronic irritation and injury to the bronchial mucosa
Infectious Processes
Viral Infection
Clinical
♦
Symptomatic patients usually present with significant symptoms; however, viral inclusions can be detected in cytologic smears from asymptomatic patients with no active disease
Microscopic
♦




General background features seen in the company of viral infections include ciliocytophthoria, necrosis, inflammation, and bronchial/alveolar cell hyperplasia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


