Current Application of Endoscopic Sphincterotomy, Lateral Choledochoduodenostomy, and Transduodenal Sphincteroplasty
E. Christopher Ellison
Jeffrey W. Hazey
W. Scott Melvin
Stephen G. Moon
Introduction
Endoscopic sphincterotomy, transduodenal sphincteroplasty, and lateral choledochoduodenostomy are complementary procedures used when dealing with distal common bile duct obstruction due to stones, strictures, or, in some cases, motor dysfunction of the Oddi sphincter. Surgical drainage procedures have, in many instances, been replaced by endoscopic papillotomy or sphincterotomy. However, there remain situations in which surgical therapy is required. Each of the procedures has specific indications, contraindications, advantages, and disadvantages. This chapter considers each procedure and discusses applications so that the surgeon can make a rational choice concerning which technique is appropriate to a given clinical circumstance. Before proceeding with discussion of the endoscopic and surgical techniques, the anatomy and physiology of the distal common bile duct and pancreatic duct are addressed.
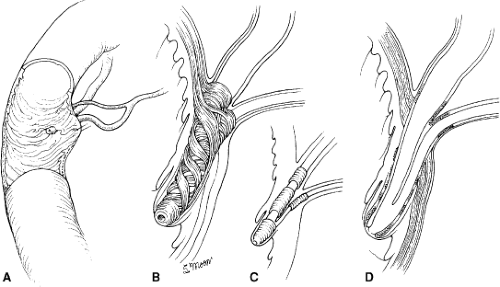
The common bile duct and main pancreatic duct run in parallel as they enter the duodenum (Fig. 1). It is important to recognize the anatomic relationship between the common bile duct and the insertion of the main pancreatic duct to avoid damage to the latter structure during sphincteroplasty. As the common bile duct courses through the muscle layers of the duodenum, its caliber tapers markedly, producing the characteristic narrowing seen during cholangiography. This tapering is the result of a complex sphincteric mechanism surrounding the distal common bile duct. The main pancreatic duct opens into the bile duct at a variable distance from the major duodenal papilla. A common channel is present in ∼85% of cases. In these situations, dissections have demonstrated that the pancreatic duct is usually located on the medial aspect of the common duct, entering 2 to 3 mm proximal to the papilla of Vater. In ∼6% of patients,
the ducts open close to one another but separately on the papilla of Vater. The pancreatic and bile ducts open into the duodenum at separate points in 9% of cases.
the ducts open close to one another but separately on the papilla of Vater. The pancreatic and bile ducts open into the duodenum at separate points in 9% of cases.
Control of the flow of bile and pancreatic juice is regulated by a complex sphincteric mechanism that consists of four sphincters of smooth muscle surrounding the intramural portion of the common bile duct, main pancreatic duct, and ampulla (Fig. 1B–D). This collection of musculature was first noted by Oddi in 1888 and was expanded upon by Boyden’s anatomic descriptions in 1936. This complex is functionally separate from the duodenal musculature. The bile duct sphincter itself consists of three interconnected sphincters: (a) the superior sphincter surrounding the bile duct for 0.5 cm just proximal to its passage through the duodenal wall, (b) a submucosal portion surrounding the intramural duct, and (c) the inferior portion, or the sphincter of the hepatopancreatic ampulla located within the ampulla itself. A pancreatic sphincter is described surrounding the intramural portion of the pancreatic duct as well. Depending on the angle at which the biliary and pancreatic ducts pass through the duodenal wall, the total sphincteric complex may be as short as 6 mm or as long as 30 mm.
Physiology of the Oddi Sphincter
Physiologic studies have demonstrated that the Oddi sphincter generates a resistance of ∼5 mm Hg at the opening of the bile duct and pancreatic duct. The basal pressure measured manometrically is usually 5 to 15 mm Hg greater than the common bile duct pressure and 15 to 30 mm Hg greater than the duodenal pressure. Superimposed on this tonal pressure are prominent phasic contractions (100 to 150 mm Hg), which occur at a frequency of 2 to 6 per minute and are propagated in an anterograde direction (from bile duct to duodenum). Bile flows mainly between these phasic contractions through a relaxed musculature. However, the contractions do propel a small bolus of fluid, allowing clearance of the ductal opening, but the major portion of bile flow occurs by passive means.
The frequency of contractions of the Oddi sphincter varies between the fed and fasted state. During fasting, contractions of the sphincter increase just before phase III duodenal activity. After feeding, the amplitude of contractions decreases, and the tone is reduced. This facilitates passive bile flow. Neuronal and hormonal mechanisms are involved with the fine-tuning of sphincteric contractions, yet many of the precise details of this interplay have not been fully elucidated.
Pathophysiology
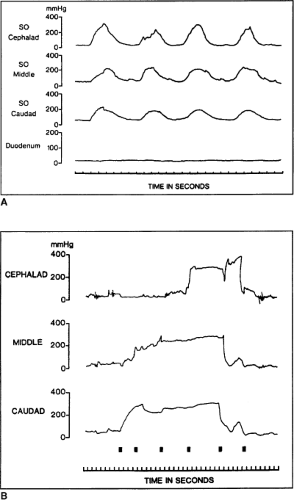
Successful miniaturization of manometry catheters, so that they can be used through side-viewing endoscopes, has permitted measurement of Oddi sphincter pressures. This has not only enhanced our understanding of normal physiology, but has also allowed accurate manometric definition of disorders of the sphincter complex. These triple-lumen catheters are either made of polyethylene or teflon, with an outer diameter of 1.5 to 1.7 mm. Side holes are oriented so that the lumens record across a length of 5 mm. The catheters are placed with a side-viewing scope before other instrumentation of the papilla. A patient undergoing endoscopic Oddi sphincter manometry is prepared somewhat differently than for standard endoscopic retrograde cholangiopancreatography (ERCP). After topical anesthesia of the oropharynx, mild sedation is achieved with intravenous diazepam or midazolam. It is important to avoid anticholinergics, such as atropine, or opiate analgesics, such as morphine, as they reproducibly alter Oddi sphincter pressures. Figure 2A shows a normal motility of the sphincter. Four manometric abnormalities have been categorized. The first abnormality is increased basal pressure in the Oddi sphincter (basal pressure >40 mm Hg). This abnormality has been further subdivided into two entities: (a) papillary stenosis, which occurs if the increased basal pressure does not relax after the administration of smooth-muscle relaxants such as nitrates, and (b) dyskinesia, when the basal pressure does respond to smooth-muscle relaxants. The second condition is tachyoddia and is evidenced manometrically by an increased number of phasic contractions (>38 per minute). The third abnormality is an increased number of retrograde waves (from duodenum to bile duct), with a corresponding decrease in the number of anterograde waves. The fourth abnormality is a paradoxic response to cholecystokinin (CCK)-octapeptide. Ordinarily, CCK-octapeptide, when given intravenously, should cause a decrease in Oddi sphincter basal pressure and phasic wave pressures. The paradoxic response occurs when CCK causes an increase in basal and phasic wave pressures.
These classifications of motility disorders correlate with a spectrum of clinical syndromes. It is postulated that disordered motility causes poor emptying of the biliary tree, ductal dilation, and pain. Most patients have had cholecystectomy, and the symptoms are nearly identical to those produced by cholelithiasis and dyskinesia of the gallbladder. Abdominal pain can be constant or intermittent and may not be temporally associated with dietary intake. Elevation of liver function tests is often seen in these disorders, but the abnormalities may be mild and, in some patients, transient, correlating with their episodes of pain.
Sphincter of Oddi Dysfunction and Endoscopic Sphincterotomy
A number of patients have sphincter of Oddi dysfunction (SOD). These patients present with intermittent biliary- or
pancreatic-type pain after cholecystectomy or in the absence of gallstones and without significant “dyskinesia” determined by CCK-HIDA scan. Similarly, idiopathic bouts of acute pancreatitis or pancreatic-type pain in the absence of alcohol ingestion, gallstones, or other risk factors remain an enigma to the clinician. Transient elevations of biliary or pancreatic enzymes may occur in the presence or absence of pain. This may be best assessed with biliary manometry performed utilizing a side-viewing duodenoscope and inserting a manometric catheter through the ampulla selectively into the bile or pancreatic duct. Sphincter of Oddi manometry is quickly becoming the “gold standard” to assess Oddi function. Sphincters with an elevated basal pressure or inappropriately contracting may correlate with the patient’s pain and respond to sphincterotomy. New evidence indicates that patients with normal manometric findings and persistent symptoms may benefit from repeat manometry as some patients may have periodic abnormalities in biliary manometry. Endoscopic sphincterotomy is quickly replacing surgical sphincteroplasty as the treatment of choice for SOD.
pancreatic-type pain after cholecystectomy or in the absence of gallstones and without significant “dyskinesia” determined by CCK-HIDA scan. Similarly, idiopathic bouts of acute pancreatitis or pancreatic-type pain in the absence of alcohol ingestion, gallstones, or other risk factors remain an enigma to the clinician. Transient elevations of biliary or pancreatic enzymes may occur in the presence or absence of pain. This may be best assessed with biliary manometry performed utilizing a side-viewing duodenoscope and inserting a manometric catheter through the ampulla selectively into the bile or pancreatic duct. Sphincter of Oddi manometry is quickly becoming the “gold standard” to assess Oddi function. Sphincters with an elevated basal pressure or inappropriately contracting may correlate with the patient’s pain and respond to sphincterotomy. New evidence indicates that patients with normal manometric findings and persistent symptoms may benefit from repeat manometry as some patients may have periodic abnormalities in biliary manometry. Endoscopic sphincterotomy is quickly replacing surgical sphincteroplasty as the treatment of choice for SOD.
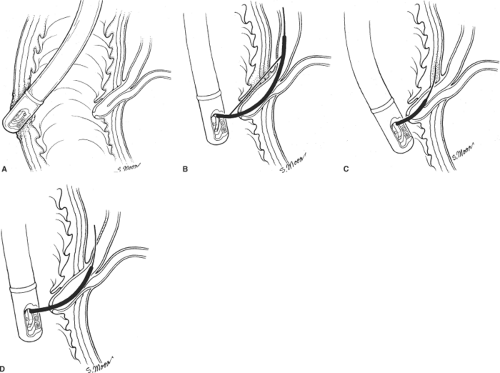
As indicated above, patients with SOD or sphincter of Oddi stenosis may present postcholecystectomy or after an exhaustive workup for recurrent idiopathic pancreatitis or biliary-type pain in the context of a normal right upper quadrant ultrasound and a normal CCK-HIDA scan. Patients may have no biliary dilation or increased enzymes that correlate with episodes of pain. Sphincter of Oddi manometry is helpful in such cases. As previously indicated, the sphincter of Oddi is a complex smooth-muscle mechanism with sphincteric musculature involving both the bile and the pancreatic duct (Fig. 3A). Manometry can measure the transsphincteric basal and contractile pressures across both systems. Basal pressures >40 mm Hg, ductal pressures >13 mm Hg, and phasic contractions >220 mm Hg for >8 seconds at a frequency of >10 per minute suggest abnormal manometry of either the biliary or the pancreatic sphincter mechanism. The most frequent complication seen with sphincter of Oddi manometry, pancreatitis, is decreasing in frequency with the introduction of nonperfused pressure catheters. Endoscopic sphincterotomy of the biliary, pancreatic, or both sphincteric mechanisms may be necessary to render the sphincter incompetent and alleviate a patient’s pain (Fig. 3B, C). The Geenen–Hogan sphincter of Oddi classification system has been applied to SOD as a predictor of which patients will respond to sphincterotomy. Table 2 Type I SOD comprises patients with biliary-type pain, abnormal serum glutamic-oxaloacetic transaminase (SGOT) or alkaline phosphatase more than two times normal on two or more occasions, common bile duct diameter >12 mm, and delayed drainage of contrast from the biliary tree after ERCP of >45 minutes. Patients who fulfill these criteria frequently have abnormal manometry (75% to 95%) and have a 90% to 95% symptomatic response to sphincterotomy, making manometry unnecessary in this group of patients. Type II SOD comprises patients who exhibit biliary-type pain and present with one or two of the previous criteria. Abnormal manometry is found in 55% to 65% of these patients. Sphincter of Oddi manometry is generally recommended in this population and symptomatic relief can be expected in up to 85% in the presence of abnormal manometry. In the face of normal manometry, only 35% of patients can be expected to have relief. Type III SOD patients have biliary-type pain but no other abnormalities. Sphincter of Oddi manometry is abnormal in only 25% to 60% of these patients. Similarly, symptomatic relief can be expected in 55% to 65% of patients with abnormal manometry, but <10% of these patients with normal manometry will respond. To date, this is the only predictive model for patients with SOD as it applies to symptomatic responses given in subjective clinical data and manometric values. Endoscopic sphincterotomy may be applied selectively to either the biliary or pancreatic duct sphincteric mechanism, depending on manometric findings.
The ability to perform selective or dual sphincterotomy has increased success rates and symptomatic response in patients with SOD and recurrent idiopathic pancreatitis (Table 1).
The ability to perform selective or dual sphincterotomy has increased success rates and symptomatic response in patients with SOD and recurrent idiopathic pancreatitis (Table 1).
Patients undergoing endoscopic sphincterotomy for SOD have been found to have increased complication rates when compared to those undergoing sphincterotomy for common bile duct stones. Trials of biliary and pancreatic duct stenting to aid in the diagnosis are generally not recommended with complication rates as high as 38%. The most common complication, pancreatitis, can be expected in up to 7% of all patients undergoing ERCP. Multiple risk factors have been outlined that increase the risk of developing post-ERCP pancreatitis. These include a prior history of ERCP-induced pancreatitis, balloon dilation of the sphincter, difficult cannulation, sphincterotomy, pancreatic duct injection, especially with acinarization, SOD, female gender, and younger age. The decision to alternatively offer these patients endoscopic ultrasound or magnetic resonance cholangiopancreatography (MRCP) may be influenced by these risk factors.
Complications of Ercp, Endoscopic Sphincterotomy, and Treatment
The surgeon may be consulted for a patient who develops bleeding, perforation, or stricture after ERCP with sphincterotomy. Bleeding occurs in 2% to 12% of patients who undergo sphincterotomy. This may be minor and self-limited or major, requiring laparotomy and oversewing of bleeding vessels. The majority of cases can be treated utilizing endoscopic modalities, including injection of 1:10,000 epinephrine, thermal therapies, or endoscopic hemoclips.
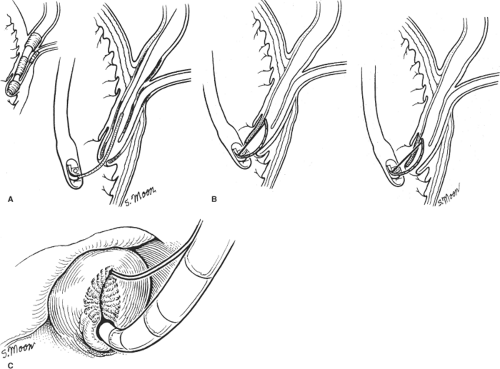 Fig. 3. A: Sphincter of Oddi musculature and biliary cannulation. B: Deployment of wire in preparation for sphincterotomy. C: Endoscopic view of completed sphincterotomy. |
Table 1 Modified Milwaukee Classification System for Sphincter of Oddi Dysfunction (Assuming all Patients have Biliary Type Pain) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Perforation rates of 0.3% to 1.0% have been reported. Perforations may occur as a
result of endoscopic manipulation independent of ERCP or as a result of sphincterotomy or passage of a guidewire. Treatment of perforations as a result of sphincterotomy depends on location and have been categorized as type I through type IV by Stapfer et al. (Fig. 4). Type I perforations involve the lateral or medial wall of the duodenum and are caused by the endoscope. There is often free intraperitoneal extravasation of contrast from the duodenum, and these perforations require laparotomy and repair. Duodenal diversion may be necessary depending on the extent of the injury. Type II perforations, or peri-Vaterian injuries, may be diagnosed during the procedure or with a computed tomography scan with oral contrast. These perforations vary in severity, tend to be small, and are less likely to require surgery. When surgical repair is indicated, stone/foreign body removal, drainage of the periduodenum and, rarely, duodenal diversion may be necessary. Type III perforations involve only the bile duct and tend to be small. Although these perforations may be diagnosed during the procedure, it is not uncommon for them to be diagnosed after ERCP. The bile duct may be perforated as a result of sphincterotomy, passage of a guidewire, or bile duct manipulation. Conservative treatment with bowel rest, intravenous antibiotics, and either endoscopic stenting or nasobiliary drainage are often successful. In those patients who develop a biloma or an intraperitoneal collection of bile, external percutaneous biliary drainage in combination with internal stenting is generally successful. Free intraperitoneal bile leaks that are not amenable to percutaneous drainage may require laparotomy, washout, and placement of drains. The need for primary operative repair of biliary injuries/leaks in this setting is unusual. Type IV perforations are associated with retroperitoneal air and no free extravasation of contrast. These are usually limited to the retroperitoneal duodenum. Series report successful treatment in up to 86% of these patients with bowel rest, nasogastric decompression, and broad-spectrum antibiotics. Complex injuries involving both the duodenum and the biliary tree may necessitate more complex treatment algorithms. Using a combination of the above described techniques, surgical intervention may be unnecessary. Postsphincterotomy strictures do occur and may be amenable to repeat sphincterotomy or balloon dilation. In rare circumstances, recurrent stricturing of the ampulla may require operative transduodenal sphincteroplasty.
result of endoscopic manipulation independent of ERCP or as a result of sphincterotomy or passage of a guidewire. Treatment of perforations as a result of sphincterotomy depends on location and have been categorized as type I through type IV by Stapfer et al. (Fig. 4). Type I perforations involve the lateral or medial wall of the duodenum and are caused by the endoscope. There is often free intraperitoneal extravasation of contrast from the duodenum, and these perforations require laparotomy and repair. Duodenal diversion may be necessary depending on the extent of the injury. Type II perforations, or peri-Vaterian injuries, may be diagnosed during the procedure or with a computed tomography scan with oral contrast. These perforations vary in severity, tend to be small, and are less likely to require surgery. When surgical repair is indicated, stone/foreign body removal, drainage of the periduodenum and, rarely, duodenal diversion may be necessary. Type III perforations involve only the bile duct and tend to be small. Although these perforations may be diagnosed during the procedure, it is not uncommon for them to be diagnosed after ERCP. The bile duct may be perforated as a result of sphincterotomy, passage of a guidewire, or bile duct manipulation. Conservative treatment with bowel rest, intravenous antibiotics, and either endoscopic stenting or nasobiliary drainage are often successful. In those patients who develop a biloma or an intraperitoneal collection of bile, external percutaneous biliary drainage in combination with internal stenting is generally successful. Free intraperitoneal bile leaks that are not amenable to percutaneous drainage may require laparotomy, washout, and placement of drains. The need for primary operative repair of biliary injuries/leaks in this setting is unusual. Type IV perforations are associated with retroperitoneal air and no free extravasation of contrast. These are usually limited to the retroperitoneal duodenum. Series report successful treatment in up to 86% of these patients with bowel rest, nasogastric decompression, and broad-spectrum antibiotics. Complex injuries involving both the duodenum and the biliary tree may necessitate more complex treatment algorithms. Using a combination of the above described techniques, surgical intervention may be unnecessary. Postsphincterotomy strictures do occur and may be amenable to repeat sphincterotomy or balloon dilation. In rare circumstances, recurrent stricturing of the ampulla may require operative transduodenal sphincteroplasty.
Infectious complications of ERCP include cholangitis (1% or less) and cholecystitis (0.2% to 0.5%). Placement of stents, the presence of strictures, jaundice, and incomplete biliary drainage increase the risk of cholangitis. Drainage of the biliary tree by stenting or sphincterotomy with antibiotics is the treatment of choice. The presence of gallstones is believed to be the causal factor in post-ERCP cholecystitis and can be treated with cholecystectomy.
Lateral Choledochoduodenostomy
Lateral choledochoduodenostomy can be performed as a primary procedure at the time of cholecystectomy or for recurrent common bile duct stones occurring remotely after cholecystectomy that cannot be treated by endoscopic papillotomy. In the past, the frequency of the necessity of this drainage procedure has been ∼1% of all biliary operations, 38% of patients having a primary operation and 60% having a secondary procedure. It is more often necessary in the elderly; the mean age of patients reported in the literature is 61 years.