Crohn Disease
Alexandros D. Polydorides, MD, PhD
Key Facts
Terminology
IBD discontinuously involving upper or lower GI
Etiology/Pathogenesis
Environmental trigger in genetically predisposed individuals leads to immunologically mediated injury
Clinical Issues
Peak: 2nd-3rd decade (smaller peak: 6th-7th decade)
Ileocolonic, small bowel only, or colon only
5-30% of CD patients have upper GI disease
Abdominal pain, diarrhea, bleeding, obstruction
Most patients undergo surgery at some point
Drugs: Aminosalicylates, immunomodulators, antibiotics, corticosteroids
Recurrence: 94% within 10 years of diagnosis
Dysplasia/cancer: ˜ 5% patients develop GI cancer
Macroscopic Features
Skip lesions (uninvolved bowel), abrupt transition
Small aphthous or larger serpiginous/linear ulcers
“Cobblestone” mucosa, inflammatory pseudopolyps
Fat wrapping, mesenteric adhesions, abscesses
Sinus tracts, fistulas to other organs, skin
Microscopic Pathology
Basal lymphoplasmacytosis, crypt branching/dropout
Crypt abscesses, polypoid granulation tissue
Paneth cell and pseudopyloric gland metaplasia
Fissuring ulcers to muscularis propria
Transmural lymphoid aggregates
Submucosal fibrosis, muscle and nerve hypertrophy
Large, well-formed epithelioid cell granulomas
Mostly in submucosa, subserosa; ↑ distally
TERMINOLOGY
Abbreviations
Crohn disease (CD)
Inflammatory bowel disease (IBD)
Crohn colitis (CC)
Definitions
IBD = CD and ulcerative colitis (UC)
Involves any part of GI tract, mouth to anus
Characterized by patchy segmental transmural chronic inflammation with granulomas and associated fissures, fibrosis, neuromuscular hypertrophy, strictures, fistulas
ETIOLOGY/PATHOGENESIS
Environmental Exposure
Possible gut-specific sensitization to food antigens
Smoking: Exacerbates CD, ↑ risk of recurrence, relapse
Diet: Zinc deficiency may cause immune dysfunction
Elemental diet: ↓ intestinal permeability, symptoms
Increasing CD incidence in developing world
Better hygiene, vaccination, ↓ pathogen exposure
Possibly due to inadequate development of mucosal immunity
Infectious Agents
CD patients: ↑ incidence of childhood infections
↑ serum levels of antibodies against enteric organisms
Mycobacterium paratuberculosis: Detected by PCR in some cases
Also implicated: Measles, Yersinia, indigenous flora
Genetic Predisposition
Clustering: Higher incidence in Ashkenazi Jews, Welsh
Familial risk: 10-25x higher incidence in 1° relatives
5-10% of IBD patients have affected relatives
25% of families: Discordant IBD type (CD vs. UC)
Suggests common & distinct genetic susceptibilities
Concordance: Mono- (50%) > di-zygotic (5%) twins
Significantly higher concordance rates than UC
Incomplete penetrance suggests additional factors
Polymorphisms (mutations) in NOD2 (CARD15)
Expressed in monocytes and enterocytes
Recognizes bacterial peptidoglycan, activates NF-κB
Mutations cause defective response to peptidoglycan
Ineffective innate, overactive adaptive immunity
↑ bacterial survival, ↑ chronic inflammation
Maps to IBD1 susceptibility locus (chromosome 16)
Linkage to CD only (early onset, severe), not UC
Immunologic Factors
IBD: Disease of abnormal immune response regulation
Both innate (macrophages, neutrophils) and adaptive (lymphocytes, plasma cells) immunity
Excess stimulation (proinflammatory cytokines)
Upregulation: T-cell activation, antibody production
↓ tolerance to luminal antigens, enteric commensals
Bacterial cell wall products, metabolites, and toxins
CD patients may have ↑ intestinal permeability
↑ antigen absorption, bacterial invasion of mucosa
Increased activated dendritic cells in lamina propria
Toll-like receptors activate downstream NF-κB
↑ TNF, IL-1β expression (pro-inflammatory activity)
Hyperactive CD4(+) T-cell response to normal flora
↑ TH1 cytokine (IFN-γ, IL-12, IL-17) production
↑ IgG production against commensal organisms
IBD flares may be ameliorated by antibiotics
Positive and negative associations with HLAs
Unknown Cause, Likely Multifactorial
Environmental trigger in genetically predisposed individuals leads to immunologically mediated injury
CLINICAL ISSUES
Epidemiology
Site
30-50%: Ileocolonic involvement
Distal 15-25 cm terminal ileum, right colon/cecum
25-40%: Small intestine involvement only
Terminal ileum only > diffuse small bowel disease
10-30%: Colonic disease only (isolated CC)
5-30% of CD patients have upper GI disease
Colonic involvement: Variable, rectal sparing in ˜ 50%
More common in adults vs. children
R > L
Longer lengths involved (30-50 cm) vs. small bowel
Presentation
Often subtle: Diagnosis delayed after symptom onset
Depends on site involved, severity of inflammation
Intermittent, crampy, postprandial abdominal pain
Watery diarrhea or loose stools, especially at night
Perforation, bleeding (fissuring mucosal ulcers), fistula
Obstruction due to strictures (especially distal ileum)
Ileocecal disease mimics appendicitis, palpable mass
Malabsorption: Vitamins (B12, fat-soluble), zinc
May lead to growth retardation in pediatric patients
Colonic disease mimics UC (bloody, mucus diarrhea)
Sudden worsening: Superimposed infection (CMV) or ischemia (vasculitis), especially in immunosuppressed
Children may have predominance of systemic and extraintestinal symptoms, minimal GI signs
Endoscopic Findings
Colonoscopy with cannulation of terminal ileum
Increasing use of upper endoscopy in CD patients
Endoscopic findings do not reliably predict response
Early: Small aphthous lesions or erythematous ulcers
Late: Granularity, nodularity, “cobblestone” mucosa
Linear ulcers, rigidity of wall, stenosis, strictures
Surveillance for dysplasia/carcinoma is not as routine as it is for UC patients
Longstanding CC, strictures: Follow closely
Surveillance is difficult due to strictures
Laboratory Tests
Circulating antineutrophil cytoplasmic antibodies
Perinuclear staining pattern (p-ANCA)
Much more common and specific for UC
25% of CD patients: Positive serum p-ANCA
Tend to have left-sided disease, similar to UC
Natural History
2 forms of CD based on disease progression
Inherently indolent (nonperforating): Recurs slowly
Initially presents with inflammatory symptoms
Pain, diarrhea, weight loss, fever, GI bleeding
Exaggerated proinflammatory cytokine response
Can become fibrostenotic (obstruction, jaundice)
Inherently aggressive (perforating): Evolves rapidly
Prone to develop fistulizing disease and abscesses
Patients with small bowel disease or prior surgery
Up to 60% have internal or external fistulae
Spontaneously; to other bowel, vagina, bladder
Perforation in 1.5%
2° to ischemia, infection, or spontaneous (abscess)
Treatment
Options, risks, complications
CC/UC patients after restorative proctocolectomy
Fecal stream diversion: Follicular hyperplasia, transmural inflammation, granulomas, fissures (difficult to distinguish from CD recurrence)
Ileostomy: Inflammatory polyps, prolapse, ulcers
Aphthous ulcer: 1st CD manifestation in neoileum
Pouchitis: Role of bacterial overgrowth from stasis
Resembles CD: Distortion, Paneth cell hyperplasia
Evaluate transmural disease far from anastomosis
Toxic megacolon: Mostly transverse, but not only
Complicates CD, UC; also seen in ischemic colitis and C. difficile colitis
Diameter > 6 cm without mechanical obstruction
Acutely ill patient: Fever, leukocytosis, distention
Mortality (25%), perforation → emergency surgery
Mucosal stripping, necrotic muscle with fissuring
Surgical approaches
Most patients will undergo surgery at some point
20% in 1st year of diagnosis, 5% per year after
> 60%: Repeated operations → risk of short bowel
Balance: Disease resection vs. intestinal function
Indications: Abscess, fistula, bleeding, perforation, obstruction, intractable disease, dysplasia/carcinoma
Postoperative recurrence affected by
Indication, extent of disease at time of surgery
Smoking, failed therapy, perforating disease
Drugs
Inhibit inflammation, immune-mediated injury
Immunosuppression (cyclosporine), corticosteroids
Infection (CMV, C. difficile), osteonecrosis
Aminosalicylates (sulfasalazine, mesalazine)
Pneumonitis, hemolytic anemia, nephritis
Immunomodulators (azathioprine, mercaptopurine)
Lymphoproliferative disorders
Antibiotics (metronidazole, ciprofloxacin)
Prognosis
Recurrence common: 94% within 10 years of diagnosis
Mostly with ileocolonic disease or after surgery
Isolated ileal disease: Proximal to anastomosis
Ileocolitis, CC: Both sides of anastomosis
Regional jejunitis after surgery for ileal CD
Severe, sometimes fatal pattern of CD recurrence
Dysplasia/cancer: ˜ 5% patients develop GI carcinoma
May be multiple, usually preceded by dysplasia
Risk correlates with duration, anatomic extent
Median 15 years after diagnosis (75% > 8 years)
Risk of colorectal cancer reportedly less than in UC
Depends on extent, may be similar in cases of CC
Small bowel cancer: 10-20x ↑ risk (mostly distal)
Risk factors: > 20 years of disease, chronic fistulas
Poor prognosis: Difficult diagnosis, usually late
↑ risk of anal squamous cell carcinoma, lymphoma
IMAGE FINDINGS
Radiographic Findings
Aphthous/deep ulcers, cobblestoning, fissures, fistulas, strictures, patchy involvement: Suggest CD over UC
Poor correlation with disease severity, activity
CT Findings
Useful to evaluate thickness, abscess, stricture, mass
MACROSCOPIC FEATURES
General Features
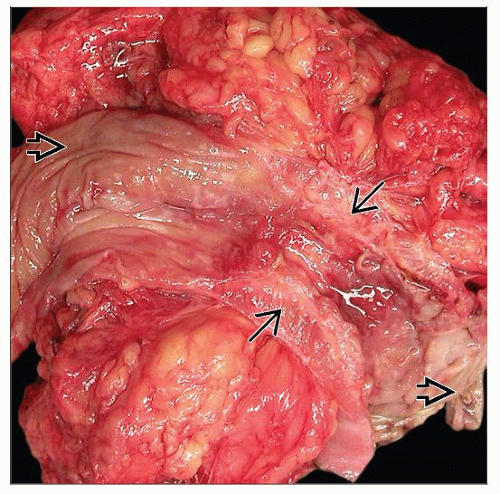
Skip lesions (uninvolved bowel) with abrupt transition
Intestinal mucosa
Small stellate aphthous ulcers
Erosions over lymphoid aggregates
Coalesce into “bear claw,” serpiginous, linear ulcers
Cobblestoning: Ulcers between uninvolved mucosa
Inflammatory pseudopolyps: Usually in transverse colon
Can be large (5 cm) or tall and narrow (filiform)
Intestinal wall
Sharp fissuring ulcers: Extend to muscularis propria
May lead to adhesions, fistulas, abscess
Submucosal fibrosis, muscular hypertrophy
Thick, firm, rigid bowel wall; can lead to stricture
Intestinal serosa
Exudate, serositis, many small nodules (granulomas)
Adhesions to other bowel, abdominal/pelvic organs
Fat wrapping (creeping fat substitution)
Adipose tissue expands toward antimesenteric surface
Partly or completely surrounds bowel
Associated with chronic transmural inflammation
Intramural or mesenteric abscesses and sinus tracts
Fistulas between organs, to skin (enterocutaneous)
Lymph nodes: Enlarged gray specks (granulomas)
Superficial CD
Classic CD but limited to mucosa and submucosa
Rare; diagnosis requires typical CD elsewhere
Minimal transmural inflammation
Bowel wall thin, pliable; strictures do not develop
In colon, superficial CC resembles UC
Extraintestinal Manifestations
Esophageal CD
Rare: < 1% of CD, less than oropharyngeal disease
Diagnosis of exclusion: Need evidence of distal CD
Aphthous ulcers, erosions, nonspecific esophagitis
Irregular stenosis, strictures: Simulate carcinoma
Gastric disease: Typically distal stomach
Antral stenosis and pyloric obstruction → vomiting
Diagnose only with concurrent intestinal disease
Important to exclude Helicobacter pylori gastritis
May present just as focally enhanced gastritis
Lymphocytes, neutrophils around single crypts
Isolated granulomatous gastritis: Early gastric CD?
Duodenal CD
Most have concomitant distal ileal or colonic disease
Can extend proximal (stomach), distal (jejunum)
Rarely, duodenum is only site involved by CD
Symptoms similar to distal disease (ulcers, strictures)
Epigastric pain, hemorrhage, nausea, vomiting
Obstruction, bleeding, duodenal-enteric fistulae
30-60% of CD patients: Endoscopic/histologic involvement without upper GI symptoms
Diagnosis relies on finding patchy, segmental, inflammatory changes ± granulomas
No other cause for inflammation (peptic injury)
Focal neutrophils in lamina propria, epithelium
Intraepithelial lymphocytosis may be present, ± associated villous blunting
Distinguish from peptic duodenitis, ulcer, eosinophilic gastroenteritis, celiac disease
Gastroduodenal CD may be more common in children
Anorectal disease
Perirectal/perianal tags, scars, erosions, ulcers, blind sinus tracts, violaceous edema, fistulae, abscesses
Common in patients with colonic disease (˜ 75%)
Correlates with genital involvement (vulvar, vaginal)
May predate primary intestinal disease by years
Oral disease
20-50% of CD patients: Lips, buccal mucosa, tongue
Vesicles, aphthous ulcers, polypoid inflammation
May herald unrecognized intestinal disease
Nonalimentary tract involvement
25% of known CD patients: At least one extra-GI site
Colon involvement, longer disease predispose
Arthritis/arthralgia, ileitis, ankylosing spondylitis
Incidence varies, may predate intestinal symptoms
Hepatobiliary: Steatosis, pericholangitis, carcinoma
Primary sclerosing cholangitis: More often in UC
Skin: Erythema nodosum, pyoderma gangrenosum
Cutaneous CD: Around stoma or distant disease
Eye: Uveitis, conjunctivitis, iritis, orbital myositis
Vulva, vagina: Granulomatous inflammation
Genitourinary: Calculi, obstructive hydronephrosis
Vasculitis: Polyarteritis nodosa, giant cell arteritis
Venous thromboembolism: Hypercoagulable state
Anemia: B12 deficiency, ↓ erythropoietin, hemolysis
MICROSCOPIC PATHOLOGY
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree