Fig. 44.1 The core structure of steroid hormones is derived from the cholesterol molecule shown.
The four rings are each identified by a letter A–D, and each carbon atom by a number.
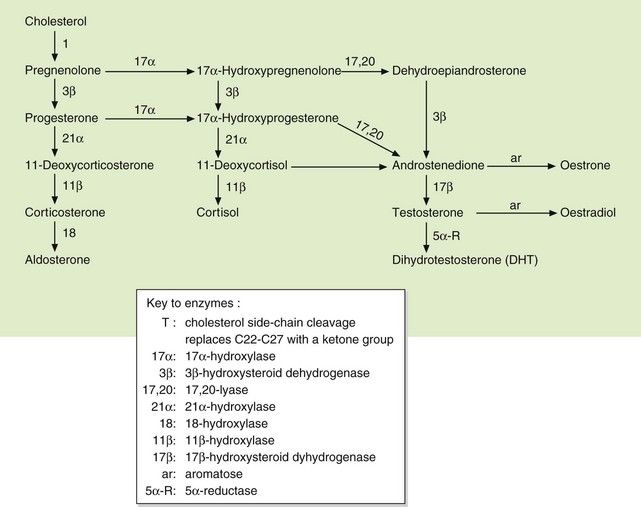
Fig. 44.2 Pathways of biosynthesis of steroid hormones, including progestogens, oestrogens, androgens, mineralocorticoids and glucocorticoids.
This chapter considers steroid hormones derived predominantly from the adrenal cortex that are known as adrenal corticosteroids. They have two distinct classes of action (see below and Table 44.1):
Table 44.1
Relative glucocorticoid and mineralocorticoid activities of some natural and synthetic corticosteroid hormones
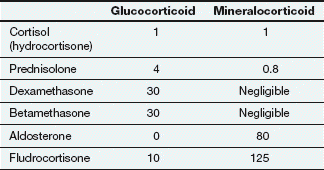
All potencies are relative to the glucocorticoid and mineralocorticoid activities of cortisol, each assigned an arbitrary value of 1. Due to intracellular metabolism by 11β-hydroxysteroid dehydrogenase in aldosterone-sensitive cells, cortisol has about one-thousandth of the mineralocorticoid activity of aldosterone in vivo.
The natural glucocorticoid is cortisol (also known as hydrocortisone), which has a hydroxyl grouping at position 17 and approximately equal affinity for glucocorticoid and mineralocorticoid receptors (but see below). The natural mineralocorticoid is aldosterone, which has an aldehyde grouping at position 18 and has little glucocorticoid activity. Synthetic corticosteroids that have been modified structurally to enhance either the glucocorticoid or mineralocorticoid activity are widely used therapeutically.
Although hydrocortisone and various synthetic derivatives are used for their glucocorticoid activity, they are frequently referred to as ‘corticosteroids’ or much less accurately as ‘steroids’. In this chapter, the distinction between glucocorticoid and mineralocorticoid is emphasised. In the rest of the book drugs with mainly glucocorticoid activity are usually referred to as corticosteroids.
Cortisol (hydrocortisone) is released from the zona fasciculata of the adrenal cortex, and its secretion is controlled by the hypothalamo–pituitary–adrenal axis (Fig. 44.3). An increase in the plasma glucocorticoid concentration feeds back negatively to the hypothalamus and pituitary to reduce the release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH; corticotropin). Glucocorticoid receptors are found in most tissues, giving cortisol a wide range of actions.
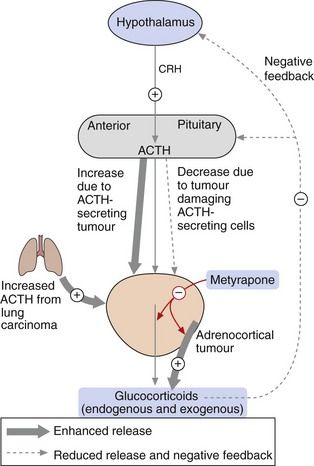
Fig. 44.3 Control of secretion of glucocorticoids.
Corticotropin-releasing hormone (CRH) from the hypothalamus stimulates adrenocorticotropic hormone (ACTH; corticotropin) from the anterior pituitary, which increases the release of glucocorticoids from the adrenal cortex. The level of glucocorticoids in the blood feeds back negatively to control the release of CRH and ACTH. Synthetic glucocorticoids have the same suppressive action on the hypothalamo–pituitary axis. In conditions in which excess glucocorticoids are released, for example in ACTH-secreting tumours or adrenocortical tumours, glucocorticoid synthesis and release can be reduced by metyrapone (red arrows). In people with tumours that result in hormone-induced reduction in glucocorticoids, synthetic glucocorticoids can be administered.
Aldosterone is secreted from the zona glomerulosa of the adrenal cortex. Aldosterone secretion is regulated by several factors, of which angiotensin II (Ch. 6), low plasma Na+ and high plasma K+ are the most important. Angiotensin II acts via specific AT1 receptors (see Chs 1 and 6) to induce aldosterone release. ACTH has a modest stimulatory effect on aldosterone secretion. Mineralocorticoid receptors are found in several tissues including the kidney, colon and heart. Important target cells are in the distal renal tubule and cortical collecting duct, where aldosterone increases the permeability of the luminal tubular membrane to Na+ by increasing the number of epithelial Na+ channels. It also stimulates the Na+/K+-ATPase pump in the basolateral membrane, which leads to active Na+ reabsorption and loss of K+ into tubular urine (Ch. 14). Water is passively reabsorbed with Na+, so that extracellular fluid volume and blood pressure are both increased. Target cells for aldosterone, especially in the renal tubule, contain 11β-hydroxysteroid dehydrogenase which metabolises cortisol to cortisone. Cortisone has very low affinity for the mineralocorticoid receptor and this ensures that most aldosterone-responsive tissues are not stimulated by endogenous glucocorticoid.
Mode of action of steroid hormones
All steroid hormones have similar intracellular receptor mechanisms, but there are distinct receptors for the different structural variants (Ch. 1). The distribution of the various receptors among tissues gives tissue specificity to each type of steroid hormone and defines its activity. In the circulation, steroid hormones are bound to specific globulins, including transcortin and sex hormone-binding globulin. Steroids are highly lipophilic and cross cell membranes by diffusion and bind to a specific cytoplasmic receptor (Ch. 1). In the absence of a steroid molecule, the receptor is retained in the cytoplasm and prevented from migrating to the cell nucleus because it is associated with a heat-shock protein (HSP). Binding of the steroid to the receptor dissociates the complex from the HSP, and the steroid–receptor complex then enters the nucleus and binds to a steroid-response element in the promoter region of the target genes (see Fig. 1.8). The binding usually involves the presence of other proteins, called chaperone proteins, and can lead to either increased or decreased transcription of proteins, depending on the target cell. Some genes are activated by simple interaction of the steroid receptor with the steroid-response element, but the rate of gene transcription is modulated by recruitment of various intranuclear co-regulator proteins and complexes.
The corticosteroid receptor only interacts with the steroid-response element for a matter of seconds before dissociating, and appears to have a ‘hit-and-run’ effect on gene transcription. When corticosteroids are given for a therapeutic effect, the response is delayed by many hours due to the time taken for modulation of protein synthesis. However, some actions of glucocorticoids are relatively rapid in onset and do not require gene transcription (non-genomic signalling pathways). They may produce direct activation of various kinases in the cell cytoplasm through a ligand-activated glucocorticoid receptor or through G-protein-coupled receptors, leading to effects such as vasodilation and regulation of cell growth.
Glucocorticoids
Actions of glucocorticoids
Immunosuppressant and anti-inflammatory actions
Glucocorticoids have important immunomodulatory actions that underpin many of their therapeutic uses. A major action of glucocorticoids is to silence pro-inflammatory genes (gene transrepression). These genes are activated by pro-inflammatory transcription factors such as nuclear factor κB (NF-κB) and activator protein 1 (AP-1) produced in response to inflammatory cytokines. NF-κB and AP-1 attach to the promoter region of pro-inflammatory genes and recruit co-activator molecules such as cAMP response element-binding protein (CBP) that have histone acetyltransferase activity. CBP acetylates core histones at the transcription complex which in turn activates pro-inflammatory gene transcription.
When glucocorticoid receptors are activated they bind to CBP at the glucocorticoid response element of inflammatory genes and inhibit histone acetyltransferase activity by recruiting co-repressor molecules. The glucocorticoid receptors also recruit histone deacetylase to suppress the pro-inflammatory genes. As a consequence, glucocorticoids inhibit the synthesis of many inflammatory cytokines, chemokines, adhesion molecules, inflammatory enzymes and receptors for inflammatory mediators.
Glucocorticoids also activate anti-inflammatory genes (gene transactivation). This is achieved by stimulation of core histone acetylation at the promoter regions of anti-inflammatory genes.
Anti-inflammatory effects
As a result of the above actions, glucocorticoids:
 reduce T-lymphocyte proliferation and increase T-cell apoptosis, which impairs cell-mediated immunity (Ch. 38). They also inhibit humoral immunity by reducing T-cell activation, B-lymphocyte proliferation and immunoglobulin production, particularly IgG (Ch. 38),
reduce T-lymphocyte proliferation and increase T-cell apoptosis, which impairs cell-mediated immunity (Ch. 38). They also inhibit humoral immunity by reducing T-cell activation, B-lymphocyte proliferation and immunoglobulin production, particularly IgG (Ch. 38), inhibit mononuclear cell and neutrophil leucocyte migration and their adhesion to inflamed capillary endothelium. The ability of these inflammatory cells to phagocytose and destroy micro-organisms and to release oxygen free radicals is also reduced through downregulation of their surface receptors (Ch. 38),
inhibit mononuclear cell and neutrophil leucocyte migration and their adhesion to inflamed capillary endothelium. The ability of these inflammatory cells to phagocytose and destroy micro-organisms and to release oxygen free radicals is also reduced through downregulation of their surface receptors (Ch. 38), reduce the synthesis of inflammatory prostaglandins by inhibition of phospholipase A2 activity (via induction of annexin-1) and by suppression of cyclo-oxygenase expression (Ch. 29),
reduce the synthesis of inflammatory prostaglandins by inhibition of phospholipase A2 activity (via induction of annexin-1) and by suppression of cyclo-oxygenase expression (Ch. 29), impair fibroblast activity with reduced collagen synthesis and inhibition of matrix metalloproteinases, which impairs wound repair,
impair fibroblast activity with reduced collagen synthesis and inhibition of matrix metalloproteinases, which impairs wound repair,Metabolic effects
 Gluconeogenesis is increased, particularly in the liver, using amino acids and glycerol from triglycerides. Storage of glycogen in the liver and, to a lesser extent, in muscle is increased, and uptake and utilisation of glucose in muscle and adipose tissue are impaired. These actions promote hyperglycaemia.
Gluconeogenesis is increased, particularly in the liver, using amino acids and glycerol from triglycerides. Storage of glycogen in the liver and, to a lesser extent, in muscle is increased, and uptake and utilisation of glucose in muscle and adipose tissue are impaired. These actions promote hyperglycaemia. Protein is degraded, particularly in muscle, to release amino acids for synthesis of glucose while protein synthesis is inhibited. As a result there is an overall negative nitrogen balance.
Protein is degraded, particularly in muscle, to release amino acids for synthesis of glucose while protein synthesis is inhibited. As a result there is an overall negative nitrogen balance.Effects on bone metabolism
 Osteoblast formation is decreased, and apoptosis of mature osteoblasts is increased. The function of mature osteoblasts is inhibited by reducing production of osteocalcin, a key extracellular matrix protein in bone that promotes bone mineralisation. Osteocyte apoptosis is increased which reduces bone strength. By contrast, survival of osteoclasts is prolonged. These actions lead to bone resorption and demineralisation.
Osteoblast formation is decreased, and apoptosis of mature osteoblasts is increased. The function of mature osteoblasts is inhibited by reducing production of osteocalcin, a key extracellular matrix protein in bone that promotes bone mineralisation. Osteocyte apoptosis is increased which reduces bone strength. By contrast, survival of osteoclasts is prolonged. These actions lead to bone resorption and demineralisation.Central nervous system effects
Plasma cortisol concentrations rise to a peak at the time of awakening and are lowest during sleep. In general, high circulating concentrations of cortisol are associated with alertness, but severe disturbances of mood may occur with abnormally high levels of glucocorticoid. Low concentrations produce a feeling of lethargy.
Mineralocorticoid effects
Natural glucocorticoids also have mineralocorticoid activity, although this has minimal impact at physiological doses (see above). Synthetic glucocorticoid compounds are altered structurally to minimise the amount of mineralocorticoid activity (Table 44.1).
Pharmacokinetics of glucocorticoids
Both hydrocortisone and synthetic glucocorticoids are used in clinical practice. They are readily absorbed from the gut. Hydrocortisone binds to corticosteroid-binding globulin (transcortin) and to albumin in the blood, and is extensively metabolised in the gut wall and liver. Synthetic glucocorticoids are more potent than hydrocortisone and bind to albumin but not to transcortin. They are more slowly metabolised in the liver, giving them a longer duration of action. Of the many synthetic glucocorticoids, dexamethasone is the most potent and has the least mineralocorticoid activity.
Most glucocorticoids are available in formulations for parenteral use. This does not appreciably shorten the time to onset of action, since most effects are delayed by up to 8 h while protein synthesis is modulated intracellularly. Some glucocorticoids are available in formulations for topical use (e.g. beclometasone, budesonide and fluticasone by inhaler for asthma). This reduces their systemic actions although systemic unwanted effects can still occur, particularly with high doses (see also Chs 12 and 34).
The plasma half-lives of glucocorticoids vary, but, because their mechanism of action depend on gene transcription and changes in protein synthesis, their biological (i.e. effective) half-lives are long (varying from 8 h for hydrocortisone to 2 days for dexamethasone).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







