Fig. 3.1
Schematic representation of pituitary suppression regimens in IVF. (a) Long GnRH agonist regimen. (b) Short GnRH agonist regimen. (c) GnRH antagonist regimen
Although early studies suggested the agonist regimen to be superior to antagonist regimen [15], later evidence suggested comparable pregnancy rates with the agonist and antagonist regimens [16]. The antagonist regimen is associated with a lower risk of ovarian OHSS and lower gonadotrophin consumption compared to the agonist regimen [16]. Between the long and the short GnRH agonist regimens, the long regimen has better outcomes in terms of the number of oocytes retrieved and pregnancy rates compared to the short regimen [17]. The GnRH antagonist and long GnRH agonist regimens are therefore suitable options for pituitary downregulation in unselected women.
A survey conducted in 2010 involving 196 centres from 45 countries showed a wide variation in the GnRH analogue regimens chosen for poor responders [18]. A recent randomised controlled trial comparing the long GnRH agonist regimen versus short GnRH agonist regimen versus GnRH antagonist regimen in women with a previous poor ovarian response demonstrated the long agonist and antagonist regimens to be suitable for these women with regard to the number of oocytes retrieved [19]. A worldwide survey in 2010 involving 179,300 IVF cycles from 262 centres in 68 countries showed the use of GnRH antagonist-based regimens in around 50 % of IVF cycles among women with polycystic ovarian syndrome (PCOS) [20]. A recent meta-analysis of studies comparing GnRH antagonist versus GnRH agonist protocols in women with PCOS involving nine RCTs from 2002 to 2013 showed comparable pregnancy rates between the two groups and a significantly lower incidence in severe OHSS in the GnRH antagonist group [21]. An added advantage with the use of GnRH antagonist-based protocols is the use of GnRH agonist trigger as a substitute for hCG in triggering of final oocyte maturation and potentially eliminating the risk of OHSS.
Ovarian Stimulation with Gonadotrophins
Gonadotrophin Dose
Exogenous gonadotrophin administration leads to supraphysiological circulating levels of FSH which facilitate recruitment of multiple follicles by exceeding the ovarian FSH sensitivity threshold [22, 23]. It is imperative to use the right gonadotrophin dose to optimise the number of oocytes retrieved and live birth rates following IVF and at the same time minimise risks such as OHSS and cycle cancellation. When exogenous gonadotrophin is administered, the number of mature follicles recruited largely depends upon the number of follicles attaining FSH sensitivity. Hence in women with a large antral follicle pool the administration of a high gonadotrophin dose may induce excessive ovarian response consequently leading to a high risk of OHSS. On the other hand, administration of an inappropriately low gonadotrophin dose may lead to the growth of a low number of follicles resulting in an ‘iatrogenic’ poor response.
An RCT comparing a gonadotrophin dose of 225 IU daily versus 150 IU daily in women aged 23–41 years undergoing IVF demonstrated the number of oocytes to be significantly higher with 225 IU daily compared to 150 IU daily [24]. This study excluded women with basal FSH > 10 IU/l, PCOS, previous poor response, and previous OHSS. Another RCT comparing gonadotrophin dose 225 IU daily versus 300 IU daily among women predicted as normal responders based on a total AFC of 8–21 showed no significant difference in the number of oocytes retrieved between the two doses [25]. This evidence would therefore suggest that the ideal gonadotrophin dose for women predicted as normal responders is 225 IU daily.
According to the worldwide survey on poor ovarian response, high gonadotrophin doses of >300 IU daily are used in around 50 % of IVF cycles for poor responders [18]. There is however no evidence to suggest that higher gonadotrophin doses result in a higher yield of oocytes and improve pregnancy outcome for poor responders. An RCT comparing gonadotrophin doses of 300 IU vs. 375 IU vs. 450 IU daily among women predicted as poor responders based on a total AFC of <12 showed no significant difference in the number of oocytes retrieved nor live birth rates between the three arms suggesting an unlikely benefit with gonadotrophin doses >300 IU daily [26]. The term hyper-response refers to the retrieval of >15 oocytes [27] or 20 oocytes [28] following conventional stimulation. It is vital to accurately predict women who are likely to have an excessive response and accordingly individualise the gonadotrophin stimulation dose to reduce the risk of OHSS. Women with PCOS and those predicted to have a hyper-response should be stimulated with a lower gonadotrophin dose of ≤150 IU daily as this will avoid excessive response. Excessive response (>20 oocytes) is also associated with a decrease in live birth rate in fresh IVF cycles [2] in addition to the higher incidence of OHSS with >18 oocytes [29–31].
Gonadotrophin Type
The successful therapeutic use of urinary gonadotrophins started with the first-generation product human menopausal gonadotrophin (hMG) or menotropin, which contained 75 IU of FSH and 75 IU of LH in each standard ampoule. This was followed in the early 1980s by the development of urofollitropin, the second-generation product from which the LH activity had been reduced to 0.1 IU/75 IU FSH [32]. Subsequently, the third-generation product, highly purified urofollitropin (Metrodin HP®) with practically no residual LH activity, was developed in the early 1990s. Due to its enhanced purity with very small amount of protein, Metrodin HP® could be administered subcutaneously which is an advantage over the previous generations which had to be administered intramuscularly. The more recent fourth-generation gonadotrophin is produced in vitro through recombinant deoxy ribo nucleic acid (DNA) technology, by genetically engineered Chinese hamster ovary cells. This is recombinant human FSH (r-FSH or follitropin) which is free of LH and contains less than 1 % of contaminant proteins [33]. There are two preparations of r-FSH that are commercially available for clinical use: follitropin-α and follitropin-β. There have been numerous RCTs comparing urinary gonadotrophins versus recombinant FSH for COS. Current evidence suggests that both the gonadotrophin preparations are comparable in IVF outcomes [34] (Fig. 3.2).
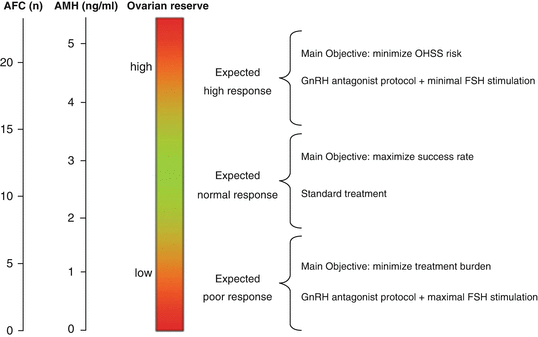

Fig. 3.2
Schematic representation of categorising women based on predicted response to individualise COS. Reproduced from La Marca & Sunkara [35]
Ovulation Trigger
Following recruitment and growth of follicles to the mature stage resulting from ovarian stimulation, the next step is maturation of oocytes facilitated by ovulation trigger in COS regimens. The LH surge that induces germinal vesicle breakdown and ovulation in a natural menstrual cycle is not reliable in stimulated multi-follicular cycles necessitating artificial triggering of ovulation. hCG which is naturally produced by the human placenta and excreted in large quantities in the urine of pregnant women bears a close molecular resemblance to LH and has a similar effect on the LH receptor. hCG can be used because of its longer serum half-life (36 h) compared to the short serum half-life of LH (108–148 min) [36], thus avoiding the inconvenience of repeated administration. Administration of hCG results in luteinisation of the granulosa cells, progesterone biosynthesis, resumption of meiosis, oocyte maturation, and subsequent follicular rupture 36–40 h later. It is administered after the stimulated development of mature preovulatory follicles in order to induce maturation, but oocyte retrieval is undertaken before ovulation. The usual criteria for the administration of hCG is the presence of ≥3 follicles of ≥18 mm in diameter. The preparations of hCG that are available for clinical use are the urinary and recombinant forms and are comparable for IVF outcomes [37]. The usual dose of hCG for final ovulation triggering is between 5,000 IU and 10,000 IU as a single dose.
The GnRH agonist trigger has been proposed as an alternative to the hCG trigger by virtue of inducing an endogenous rise in LH and FSH due to its initial flare effect [38, 39]. The GnRH agonist trigger can only be used with COS regimens where prior pituitary suppression has not been achieved with a GnRH agonist, as the mechanism of action of the GnRH agonist for downregulation and desensitisation of the pituitary receptors precludes the use of the agonist trigger. Due to the specific mode of action of the antagonist by competitive blockade of the pituitary receptors and a shorter half-life, the pituitary remains responsive to the GnRH agonist, thus enabling its use for triggering ovulation. The Cochrane review comparing the GnRH agonist versus the hCG trigger in IVF demonstrated a significantly lower incidence of OHSS and a lower live birth rate with the GnRH agonist trigger [40]. It demonstrated significantly reduced live birth rates in fresh autologous cycles with the use of the GnRH agonist trigger, but there was no reduction in live birth rates in oocyte donor/recipient cycles. Following initial use of the GnRH agonist trigger, it was soon recognised of the need to modify the standard luteal support to obtain reliable reproductive outcomes [41]. Study groups have since endeavoured to fine-tune the luteal phase support in IVF cycles using the GnRH agonist trigger to optimise clinical outcomes [42, 43]. Recent suggestions and developments in overcoming the luteal insufficiency with the GnRH agonist trigger are use of (1) a “dual trigger” [44], (2) low-dose hCG supplementation [41, 43], (3) intensive luteal oestradiol and progesterone supplementation [42], (4) rec-LH supplementation [45], and (5) luteal GnRH agonist administration [46]. A recent RCT demonstrated that an individualised luteal support based on the number of follicles following the GnRH agonist trigger optimised the pregnancy rates [47]. This study proposed ovulation triggering with 0.5 mg buserelin subcutaneously followed by a bolus of 1,500 IU of hCG after oocyte retrieval when the total number of follicles ≥11 mm was between 15 and 25 on the day of trigger and an additional 1,500 IU hCG bolus when the total number of follicles was ≤14 mm. All women received micro-ionised progesterone vaginally, 90 mg twice daily, and 4 mg of oestradiol orally commencing on the day of oocyte retrieval and continuing until 7 weeks of gestation.
Conclusion
The ultimate aim of IVF is to obtain a healthy singleton live birth with minimal adverse effects. Multiple pregnancies are recognised as a major avoidable complication of IVF. Planning of effective COS regimens is important as it leads to good quality embryos enabling selection of the best single embryo for transfer. After decades of IVF practice, it is now recognised that individualisation in IVF is the way forward. The long GnRH agonist and antagonist regimens are effective in normal responders and the ideal gonadotrophin dose is 225 IU daily. The GnRH antagonist regimen is ideal for women with PCOS and women categorised as hyper-responders. Whilst the pregnancy rates are comparable to the GnRH agonist regimen, the antagonist regimen significantly lowers the risk of OHSS in addition to enabling the use of the GnRH agonist trigger which potentially eliminates OHSS. A lower gonadotrophin dose ≤150 IU daily is recommended in these women. The long GnRH agonist and antagonist regimens are ideal for poor responders. Higher gonadotrophin doses >300 IU daily are unlikely to be beneficial in poor responders apart from higher costs and hence the maximal gonadotrophin dose should not exceed 300 IU daily.
Conflict of Interest
The author declares no conflict of interest.
References
1.
Human fertilisation and embryology authority. http://www.hfea.gov.uk/5874.html. Accessed 19 May 2014.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


