Chapter 16 Quality control
STANDARDS APPLICABLE TO CRUDE DRUGS
Preliminary examination
In the case of whole drugs the macroscopical and sensory characters are usually sufficient to enable the drug to be identified. The generalappearance of the sample will often indicate whether it is likely to comply with such standards as percentage of seed in colocynth, of ash in valerian or of matter insoluble in alcohol in asafoetida. However, drugs may comply with the descriptions given in the pharmacopoeias and yet be unsatisfactory, as it is often difficult specifically to describe deterioration of drugs owing to faulty harvesting, shipment or storage or deterioration due to age. In such cases the trained worker will be able to infer much of the history of the sample from its appearance. The following examples will serve to indicate the type of evidence to look for.
Foreign matter
The difficulty of obtaining vegetable drugs in an entirely pure condition is fully recognized, and pharmacopoeias contain statements as to the percentage of other parts of the plant or of other organic matter which may be permitted. Table 16.1 gives examples of various official types of limit applicable to specific drugs. Drugs containing appreciable quantities of potent foreign matter, animal excreta, insects or mould should, however, be rejected even though the percentage of such substances be insufficient to cause the rejection of the drug on the percentage of foreign matter.
Table 16.1 Examples of BP limits for foreign matter.
| Drugs | Foreign matter limits |
|---|---|
| Leaves and herbs | |
| Bearberry leaf |  8% Foreign matter of which 8% Foreign matter of which  5% stems and 5% stems and  3% other foreign matter. Leaves of different colour to official description 3% other foreign matter. Leaves of different colour to official description  10% 10% |
| Birch leaf |  3% Fragments of female catkins, 3% Fragments of female catkins,  3% other foreign matter 3% other foreign matter |
| Lemon balm |  10% Stems having a diameter 1 mm, 10% Stems having a diameter 1 mm,  2% other foreign matter 2% other foreign matter |
| Wild thyme |  3% Foreign matter (involves recognition of Thymus vulgaris and T. zygis) 3% Foreign matter (involves recognition of Thymus vulgaris and T. zygis) |
| Wormwood |  5% Stems with diameter greater than 4 mm, 5% Stems with diameter greater than 4 mm,  2% other foreign matter 2% other foreign matter |
| Fruits and seeds | |
| Hawthorn berries |  2% Foreign matter, 2% Foreign matter,  5% deteriorated false fruits 5% deteriorated false fruits |
| Juniper berries |  5% Unripe or discoloured cone berries, 5% Unripe or discoloured cone berries,  2% other foreign matter 2% other foreign matter |
| Psyllium seeds |  1% Foreign matter including greenish unripe fruits. No seeds of other Plantago spp. 1% Foreign matter including greenish unripe fruits. No seeds of other Plantago spp. |
| Inflorescences | |
| Calendula flowers |  5% Bracts, 5% Bracts,  2% other foreign matter 2% other foreign matter |
| Elder flowers |  8% Fragments of coarse pedicels and other foreign matter, 8% Fragments of coarse pedicels and other foreign matter,  15% discoloured brown flowers 15% discoloured brown flowers |
| Lime flowers |  2% Foreign matter, absence of other Tilia spp. 2% Foreign matter, absence of other Tilia spp. |
| Rhizomes and roots | |
| Couch grass rhizome |  15% Greyish-black pieces of rhizome in cut drug 15% Greyish-black pieces of rhizome in cut drug |
| Marshmallow root |  2% Brown deteriorated root, 2% Brown deteriorated root,  2% cork in peeled root 2% cork in peeled root |
| Valerian root |  5% Stem bases, 5% Stem bases,  2% other foreign matter 2% other foreign matter |
| Barks | |
| Quillaia bark |  2% Foreign matter 2% Foreign matter |
| Cascara bark |  1% Foreign matter 1% Foreign matter |
Moisture content
Separation and measurement of moisture
The ‘loss on drying’ methods can be made more specific for the determination of water by separating and evaluating the water obtained from a sample. This can be achieved by passing a dry inert gas through the heated sample and using an absorption train (specific for water) to collect the water carried forward; such methods can be extremely accurate, as shown in their use for the determination of hydrogen in organic compounds by traditional combustion analysis.
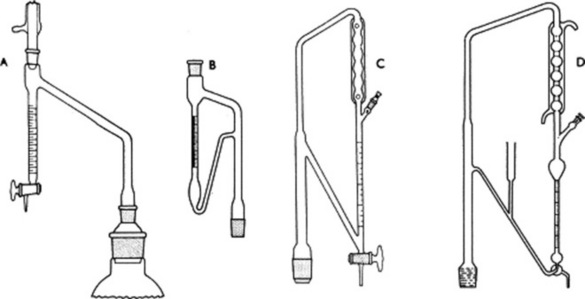
Methods based on distillation have been widely used for moisture determination. The sample to be analysed is placed in a flask together with a suitable water-saturated immiscible solvent (toluene, xylene, carbon tetrachloride) and pieces of porous pot and is distilled. The water in the sample has a considerable partial pressure and co-distils with the solvent, condensing in the distillate as an immiscible layer. A simple apparatus (Fig. 16.1A) originally devised by Dean and Stark permits the direct measurement of the water obtained and the less dense solvent (toluene, xylene) is continuously returned to the distillation flask. The method is employed in the USP and in the BP/EP for some volatile oil-containing drugs (Roman chamomile flowers, lovage root, eucalyptus, peppermint and sage leaves) and aniseed and star-anise fruits. To accommodate the loss of water due to solubility in the solvent the BP specifies a preliminary distillation of the solvent with added water (about 2 ml); the exact volume of water separating as a layer is read off and then the drug (sufficient to give a further 2–3 ml water) added to the flask and distillation resumed. Water separated from the drug is calculated from the combined final volume. Heavier-than-water solvents require the receiver shown in Fig. 16.1B. The method is readily applicable to crude drugs and food materials but has the disadvantage that relatively large quantities of the sample (5–10 g) may be required.
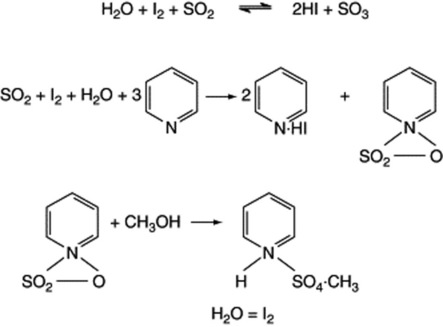
Chemical methods
In the absence of methanol, the pyridine sulphur trioxide reacts with another molecule of water. The reagent requires standardization immediately before use and this can be done by employment of a standard solution of water in methanol or by use of a hydrated salt—for example, sodium tartrate (Na2C4H4O6.2H2O). To eliminate interference from atmospheric moisture, the titration is carried out under an atmosphere of dry nitrogen, the end-point being recorded amperometrically. Equipment is now available for a completely automated determination, thus eliminating the manual aspects of sample handling and weighing, introduction to the Karl Fischer cell, titration and data completion. Although the BP Karl Fischer reagent contains pyridine, as above, the latter has been replaced by other bases (e.g. imidazoles) in some commercial reagents. Alternatives to methanol, such as diethylene glycol monoethyl ether, have been introduced to improve reagent stability.
Extractive values
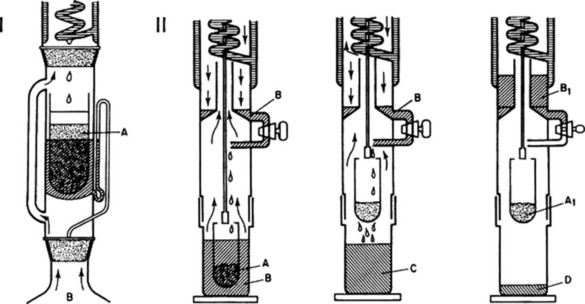
The determination of water-soluble or ethanol-soluble extractive is used as a means of evaluating drugs the constituents of which are not readily estimated by other means. But as suitable assays become available (e.g. with the anthraquinone-containing drugs), some of the previously used extractive tests are no longer required as pharmacopoeial standards. In certain cases extraction of the drug is by maceration, in others by a continuous extraction process. For the latter the Soxhlet extractor is particularly useful and has been in use for many years, not only for the determination of extractives (e.g. fixed oil in seeds) but also for small-scale isolations (Fig. 16.2). A development of the Soxhlet technique is also shown in Fig. 16.2; in this apparatus extraction is by boiling solvent followed by percolation; finally, evaporation yields the extract and the recovered solvent ready for the next sample. Some examples of the types of extractive used are given in Table 16.2.
Table 16.2 Extractives employed for drug evaluation.
| Drug | Method of evaluation |
|---|---|
| Gentian, liquorice, many drugs of BHP | Percentage of water-soluble extractive |
| Quillaia | Percentage of ethanol (45%) extractive |
| Valerian, cocillana, asafoetida | Percentage of ethanol (60%) extractive |
| Ginseng, hop strobiole | Percentage of ethanol (70%) extractive |
| Ginger, ipomoea and jalap | Percentage of ethanol (90%) extractive |
| Benzoin, catechu and Tolu balsam, myrrh | Limits of ethanol-insoluble matter |
| Colocynth | Limit of light petroleum extractive |
| Crushed linseed | Percentage of ether-soluble extractive |
Ash values
When vegetable drugs are incinerated, they leave an inorganic ash which in the case of many drugs (e.g. rhubarb) varies within fairly wide limits and is therefore of little value for purposes of evaluation. In other cases (e.g. peeled and unpeeled liquorice) the total ash figure is of importance and indicates to some extent the amount of care taken in the preparation of the drug. In the determination of total ash values the carbon must be removed at as low a temperature (450 °C) as possible without producing flames. If carbon is still present after heating at a moderate temperature, the water-soluble ash may be separated and the residue again ignited as described in the BP, or the ash may be brokenup, with the addition of alcohol, and again ignited. The total ash usually consists mainly of carbonates, phosphates, silicates and silica.
Crude fibre
The preparation of a crude fibre is a means of concentrating the more resistant cellular material of drugs for microscopical examination. It is particularly useful for rhizomes such as ginger which contain relatively large amounts of oleoresin and starch. The technique involves defatting the powder and boiling in turn with standard acid and alkali with suitable washing of the insoluble residue obtained at the different stages (see Chapter 43). The crude fibre so obtained can also be employed quantitatively to assay the fibre content of foods and animal feedstuffs and also to detect excess of certain materials in powdered drugs, e.g. clove stalk in clove. For further details see the 14th edition of this book.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree