institution. In addition, there are some business arrangements of financial support with universities or other sites for conducting part of their Phase 1 research at a specific place. It is an accepted fact that almost all Phase 1 clinical trials are outsourced, and therefore, this activity is rarely considered as a frequent issue to be discussed in terms of outsourcing.
Table 74.1 Services offered by many contract research organizations for a clinical trial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
to create an outsourcing strategy. The main components of a clinical outsourcing strategy will consider the following:
When do we believe it is most appropriate to outsource (e.g., when in-house staff are inadequate or unable to take responsibility for the trial)?
What specific types of trials do we wish to outsource (e.g., pharmacokinetic, pharmacoeconomic, Phase 1 volunteer trials, every clinical trial)?
Which specific trials in the development plan do we wish to outsource and in which phases of development (e.g., pivotal Phase 3 trials, initial Phase 2 pilot trial)?
How much of each trial do we wish to outsource (e.g., protocol writing, data management, every aspect of the clinical trial, decide on a case-by-case basis)?
Will it be better for us to use a niche CRO, a full-service CRO, a global CRO, or a managed site network?
Do we wish to create a preferred list of CROs to go to for most of our outsourcing work (i.e., all or part of the clinical development plan), or do we want to start anew for each trial, phase, or segment of the program?
How much expertise should we maintain in-house through our staff, consultants, and scientific advisory board?
Will any regulatory activities be outsourced, and if so, which?
How will we develop general performance metrics and a strategy and plan for monitoring the CRO and other vendors to ensure that all vendors are performing their services in accord with our expectations?
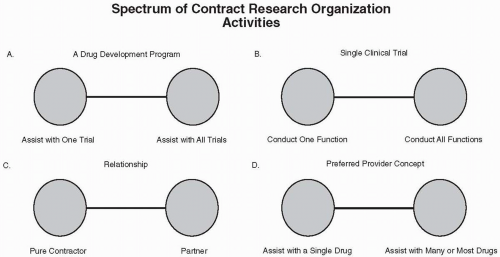 Figure 74.1 The spectrum of CRO activities in terms of several characteristics of services offered and relationships with a sponsor. |
visit reports, telephone reports, minutes of meetings, enrollment charts, project team minutes (or points of agreement and action points), and copies of e-mails. Additional information on CROs is found in Chapter 58 of Guide to Clinical Trials (Spilker 1991) and Vogel (2002).
Table 74.2 Selected forms used by contract research organizations in clinical trials | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


