Contemporary Operative Venous Thrombectomy
Anthony J. Comerota
Steven S. Gale
Introduction
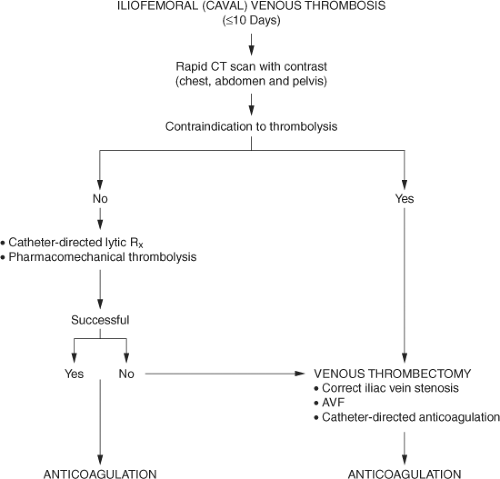
Although this is a techniques-related chapter, several background comments are warranted because of the controversy surrounding venous thrombectomy in the United States. Patients with extensive deep venous thrombosis (DVT) are at increased risk for the development of postthrombotic syndrome and this syndrome most commonly occurs in patients with the combination of venous obstruction and valvular incompetence. Moreover, anatomically, patients with iliofemoral venous obstruction in particular, with or without distal venous involvement, also suffer most severely from this disease. That seems intuitive, since the venous channel from the common femoral vein to the vena cava is a single conduit draining all blood entering the lower extremity. Intuitively, if unobstructed venous drainage from the leg into the vena cava can be restored, patients should enjoy a significantly better quality of life as a result of fewer postthrombotic sequelae. This has been shown with catheter-directed thrombolysis, as well as venous thrombectomy. Adopting a strategy of thrombus removal is an important first step in the care of these patients (Fig. 1).
Venous thrombectomy was largely abandoned in the United States because of two early reports. Interestingly, the procedures were performed approximately 50 years ago at a time when true “vascular” techniques did not exist, vascular imaging was in its infancy, and anticoagulation was inconsistent. Unfortunately, relatively recent guidelines on the management of venous thromboembolism (VTE) continued to focus on these outdated retrospective reports and overlooked more recent reports of improved outcomes using contemporary vascular techniques. Importantly, guideline authors overlooked a multicenter randomized trial evaluating contemporary venous thrombectomy versus standard anticoagulation until 2008. Results of a Scandinavian randomized trial demonstrated significant benefit to those undergoing operative venous thrombectomy, with fewer postthrombotic sequelae, increased iliac vein patency, lower venous pressures, and improved valvular function.
The technique of contemporary venous thrombectomy is designed to maximize the chance of a favorable outcome (Table 1) and is significantly different compared with the technique used in earlier reports (Table 2).
Technique of Operative Venous Thrombectomy
The principles of successful venous thrombectomy are briefly summarized in Table 1. The important conceptual elements of the procedure are numbered sequentially, with
methods to achieve these goals listed beneath each numbered item.
methods to achieve these goals listed beneath each numbered item.
Table 1 Technique of Contemporary Venous Thrombectomy | |
|---|---|
|
Patients with extensive iliofemoral venous thrombosis, which may involve the infrainguinal venous system and perhaps the vena cava, have a more aggressive thrombotic process than the majority of patients treated for acute DVT. Often, there is a reason for this degree of extensive thrombosis, which may include an underlying thrombophilia or malignancy.
The full extent of thrombus (proximally and distally) should be defined preoperatively. The infrainguinal veins are well examined by venous duplex. We prefer a contralateral iliocavagram to evaluate the vena cava and iliac veins. Magnetic resonance venography or spiral computerized tomography (CT) with contrast may obviate the cavagram in some patients.
A rapid CT scan with intravenous contrast of the head, chest, abdomen, and pelvis is commonly performed. Asymptomatic pulmonary emboli (PE) are found in approximately 50% of cases. Extending imaging from the head and chest to the abdomen and pelvis is an effective way to rapidly screen for other pathology. In addition to identifying the proximal extent of thrombus and PE, we have found patients with renal cell carcinoma, adrenal tumors, hepatic metastases, retroperitoneal lymphoma, iliac vein aneurysms, and congenital absence of the vena cava. Each of these findings had important implications for immediate and long-term patient care.
A full thrombophilia evaluation is requested upon initial examination, especially in patients with no other findings. Blood is sent for fibrinogen, antithrombin III, protein C, protein S, factor V Leiden, prothrombin gene mutation, antiphospholipid/anticardiolipin antibody, factor VIII levels, and homocysteine. If the patient is already anticoagulated, antiphospholipid/anticardiolipin antibodies, factor V Leiden, prothrombin gene mutation, and homocysteine can be reliably performed. The results of the hypercoagulable evaluation are important for appropriate recommendations regarding the duration of anticoagulation.
Therapeutic anticoagulation with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) is initiated after the blood samples are drawn for the thrombophilia evaluation. UFH is used during the procedure and early postoperative period. Although LMWH is as effective as UFH, intravenous UFH offers better temporal control of the degree of anticoagulation.
Table 2 Venous Thrombectomy: Comparison of Old and Contemporary Techniques | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
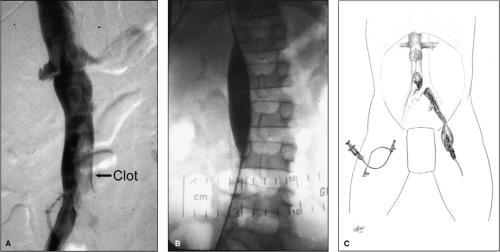
Vena caval filtration is generally not required; however, an exception may be patients with nonocclusive thrombus in the vena cava or those with symptomatic PE. Also, patients can be protected from intraoperative PE by balloon occlusion of the proximal vena cava during the operative procedure (Fig. 2). The vena caval balloon can be positioned from the contralateral femoral vein using fluoroscopic guidance during preoperative iliocavagraphy. Once the balloon is properly positioned, it remains deflated until the time of thrombus extraction. During thrombectomy, the caval balloon is inflated and the thrombectomy performed with fluoroscopic guidance, inflating all balloons with contrast mixed with saline. The operating room is always prepared for fluoroscopy with any necessary modifications in operating room table
positioning. An autotransfusion device is also available during the procedure.
positioning. An autotransfusion device is also available during the procedure.
Following the induction of general anesthesia, the entire involved leg, lower abdomen, and opposite leg to the knee are prepped into the field. In some patients both legs are prepped into the operative field. A longitudinal inguinal incision is made, exposing the common femoral vein to the inguinal ligament and saphenofemoral junction, which is dissected distally to expose the origin of the profunda femoris vein and femoral vein (Fig. 3A). A longitudinal venotomy is performed at the saphenofemoral junction. The exact location of the venotomy will depend upon the extent and location of the thrombosis. Since the femoral vein is distended, a longitudinal venotomy is preferred and can be closed primarily without compromising vein lumen.
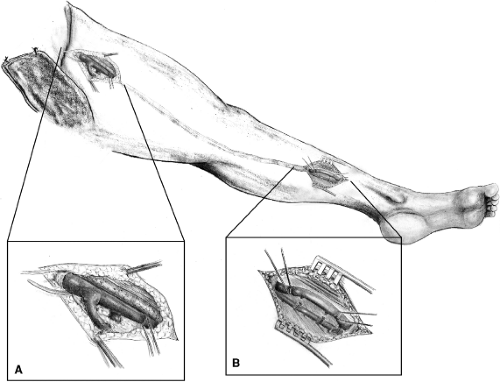 Fig. 3. A: Exposure of the common femoral, femoral, and profunda femoris veins. B: Exposure of the posterior tibial vein. |
If the infrainguinal venous system is thrombosed, an infrainguinal venous thrombectomy is performed first. Initially the leg is elevated and wrapped tightly with an Esmark. The foot is squeezed and dorsiflexed, and the leg is milked in an effort to
remove the clot from below. If these maneuvers are successful, the femoral vein below the venotomy is clamped. If unsuccessful, the distal posterior tibial vein is exposed to perform an infrainguinal venous thrombectomy (Fig. 3B). A #3 or 4 balloon catheter is passed proximally from below to exit the common femoral venotomy (Fig. 4A). The silastic sheath of a large intravenous catheter (12 to 14 gauge) is cut from its hub and slid halfway onto the balloon catheter that was passed from below. Another #4 balloon catheter is placed into the proximal end of the silastic sheath. A single operator applies pressure to the syringes attached to the two catheters, which firmly secures the balloons inside of the sheath, providing protective passage distally through the clotted venous system and valves (Fig. 4B). The catheters exit the posterior tibial venotomy (Fig. 4C). A standard balloon catheter thrombectomy can then be performed in the direction of the venous valves (Fig. 4D, E). Repeated balloon thrombectomy (with a larger catheter if necessary) can be performed until no further thrombus is extracted. At this point, the infrainguinal venous system is vigorously flushed with a heparin–saline solution to hydraulically force residual thrombus from the deep venous system (Fig. 5). This is accomplished by placing a #14 to 16 red rubber catheter into the proximal posterior tibial vein and flushing with a bulb syringe. After flushing, a vascular clamp is applied below the femoral venotomy and the infrainguinal venous system is then filled with a large volume of dilute plasminogen activator solution. Commonly, 4 to 5 mg of rt-PA is diluted in 200 mL of saline and injected into the infrainguinal venous system. If infrainguinal thrombectomy is not successful due to chronic thrombus in the femoral vein, or if there is recanalization and chronic disease of the femoral vein, it is ligated and divided just below its junction with the profunda. Patency of the profunda femoris vein must be ensured prior to ligation of the femoral vein. This maneuver prevents the inevitable venous reflux through the diseased femoral vein. Venous drainage through an unobstructed profunda system, which may have competent valves, offers a better chance at reducing postthrombotic sequelae.
remove the clot from below. If these maneuvers are successful, the femoral vein below the venotomy is clamped. If unsuccessful, the distal posterior tibial vein is exposed to perform an infrainguinal venous thrombectomy (Fig. 3B). A #3 or 4 balloon catheter is passed proximally from below to exit the common femoral venotomy (Fig. 4A). The silastic sheath of a large intravenous catheter (12 to 14 gauge) is cut from its hub and slid halfway onto the balloon catheter that was passed from below. Another #4 balloon catheter is placed into the proximal end of the silastic sheath. A single operator applies pressure to the syringes attached to the two catheters, which firmly secures the balloons inside of the sheath, providing protective passage distally through the clotted venous system and valves (Fig. 4B). The catheters exit the posterior tibial venotomy (Fig. 4C). A standard balloon catheter thrombectomy can then be performed in the direction of the venous valves (Fig. 4D, E). Repeated balloon thrombectomy (with a larger catheter if necessary) can be performed until no further thrombus is extracted. At this point, the infrainguinal venous system is vigorously flushed with a heparin–saline solution to hydraulically force residual thrombus from the deep venous system (Fig. 5). This is accomplished by placing a #14 to 16 red rubber catheter into the proximal posterior tibial vein and flushing with a bulb syringe. After flushing, a vascular clamp is applied below the femoral venotomy and the infrainguinal venous system is then filled with a large volume of dilute plasminogen activator solution. Commonly, 4 to 5 mg of rt-PA is diluted in 200 mL of saline and injected into the infrainguinal venous system. If infrainguinal thrombectomy is not successful due to chronic thrombus in the femoral vein, or if there is recanalization and chronic disease of the femoral vein, it is ligated and divided just below its junction with the profunda. Patency of the profunda femoris vein must be ensured prior to ligation of the femoral vein. This maneuver prevents the inevitable venous reflux through the diseased femoral vein. Venous drainage through an unobstructed profunda system, which may have competent valves, offers a better chance at reducing postthrombotic sequelae.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree