(1)
University of South Australia, Adelaide, Australia
Abstract
The concluding chapter presents the guide for regulators to playing and winning the new drug reimbursement game. The results from the previous chapters are summarised as four rules and four tools in this guide. Some of the counterintuitive results from applying these rules are discussed, including the result that if the new drug has additional benefits beyond health gains, that this could lead to a reduced rather that increased threshold. Likely criticisms of these rules by clinicians, social decision makers and some health economists are discussed. The regulator should not respond to these criticisms by increasing the threshold for “special cases”. Instead she should present information about the opportunity cost of the strategy to reimburse at a premium above the health shadow price to decision makers. This response goes some way in addressing the failure of the market and institutions to invest in the development of evidence of unpatented interventions. The possibility that the factor that is common to such criticism is that stakeholders value novelty per se and are willing to trade-off population health benefits to gain novelty is introduced.
11.1 The First Problem
The Reimburser looks at her original brief.
The Minister for Health and the Minister for International Trade ask the Reimburser her opinion on whether applying a decision threshold price per effect for new drugs that is lower than the FPP will lead the population’s health to be worse off in the longer run.
Her answer is that if the decision threshold is enforced at an IPER below the FPP, it is likely to lead to improved health for the population in both the short- and long-term, compared with FPP, provided that the threshold is β c . Also, lowering the price below the FPP is certain to reduce the profits to Pharma, and there is a significant incentive for Pharma to protect these rents.
Then she presents the Ministers with her reframed critical research question.
How should a rational Institution respond to the following threat by Pharma? If a purchase price for a new drug is below a FPP, this will lead to:
Suboptimal incentives for R&D;
Less new drugs in the future; and
A future population whose health will be worse that it would otherwise be.
The Reimburser is confident that, if we assume there is no relationship between price and future innovation, β c is the reimbursement decision threshold that will maximise the health of the population from current and future budgets. β c achieves this because it:
Characterises reimbursement as comprising both adoption and displacement and can therefore accommodate inefficiency arising from either or both of these actions, not just resulting from adoption as is the case with conventional CEA and
Accommodates:
Competition in the market for both R&D and current health inputs (as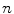 decreases so does β c );
decreases so does β c );
Inefficiency in displacement (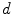 <
< 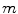 );
);
The fixed or constrained budget (there is foregone benefit); and
Allocative inefficiency (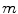 −
− 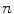 > 0) and technical inefficiency (
> 0) and technical inefficiency (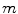 −
− 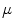 > 0).
> 0).
Furthermore, the use of β c addresses the market’s failure to develop evidence of: (1) unpatented or unpatentable services; or (2) services that will be displaced if evidence of their ICER or IPER is developed (because they are cost ineffective). The use of β c achieves this by placing an economic (decision) value on the following:
Of current services (in contraction), m;
The most cost-effective of current services (in expansion), n;
The most cost-effective investment strategy μ; and
The ICER or IPER of services that are displaced, d.
The Reimburser is also confident to state that the evidence of a positive relationship between price and future innovation is not sufficient to establish a case for pricing at the FPP. Furthermore, she has learnt to ask for the derivation of an FPP when firms claim it is the price that will maximise the population’s health.
The Reimburser is not willing to say: “There is no situation in which a drug should be reimbursed at a price higher than β c , in order to account for the relationship between price and innovation.” Only two specific reasons why the Reimburser should pay the FPP or a premium were assessed by the Health Economic Adviser (Games 2 and 3). There is no doubt that Pharma will continue to generate more win-win reasons for the FPP. Pharma is behaving exactly as we would expect a large industry protecting its economic rent to behave. However, the Reimburser now has two tools to help her assess any argument put forward by Pharma. The first tool is to analyse the problem as a GTM and not a DTM. The second tool is a range of parameters that are relevant to assessing the question of pricing higher than β c including: the IMER of current and future drugs; ΔE P of the future drug; and the uncertainty surrounding these estimates. She is, however, quietly confident that there is no case that Pharma can present that would result in a premium above β c . Her confidence has two sources. First, there is significant uncertainty in the characteristics of a future drug, even if it is already in phase 3 clinical trials. Second, the fact that the Institution is more risk-averse than the Capital Market means that Pharma will always prefer the Capital Market option if the Institution seeks a return on its investment in R&D via higher prices that compensates for the associated risk.
Finally, the Reimburser is keen to apply β c as the new drug decision threshold as soon as possible so that she can redress:
The long-term failure of the Institution to correct for the failure of the market to provide incentives for the development on unpatented or unpatentable technologies; and
The additional distortions introduced by generating incentives for firms to price drugs over the economic value of the clinical innovation at the maxWTP.
She is reminded of Arrow (1963):
The social adjustment towards optimality thus puts obstacles in its own path. (p. 947)
11.2 It’s About the Journey
The Reimburser notes the other original concepts introduced during her “Adventures in Pharma-land” and realises that to arrive at the simple result of β c as the maximum acceptable IPER regardless of the relationship between price today and innovation, many smaller simple problems needed to be solved.
With hindsight, she and the Health Economic Adviser realise that seven references were critical to the development of PEA and the health shadow price.
1.
Danzig (1963) identifies the competitive nature of an input market, even though a producer of a specific input is a monopolist. The idea that the firm must be paid the maxWTP (appropriate the entire consumer surplus without reference to competition) in order to generate appropriate incentives for R&D neglects this aspect of the economics of the competitive market.
2.
McKean (1972) explains, in words, a number of options for calculating a shadow price, and what these options mean.
3.
Comanor (1986) clarifies that the political economy of new drugs is dynamic and defines the research agenda, in terms of both inclusion and exclusion criteria.
4.
5.
Birch and Gafni (1993) reminds the health economics profession of the centrality of the concept of opportunity cost in the application of economic evaluation to decision making. Ignore it at patients’ peril.
6.
Mishan and Quah (2007) explains the difference between the shadow price of the budget constraint and the shadow price in CBA.
7.
Buchanan (2008) reminds economists of the difference between the operational definition of the counterfactual (alternative strategies available to the decision maker) and the economic concept (the best alternative end state, even if the strategy to achieve this is not directly available to the decision maker).2
The smaller problems solved on this journey relate to both pharma-economics and pharmaco-economics.
The issues addressed in relation to pharma-economics follow.
The recognition that the claim by Pharma that the population will be worse off if prices are lowered is a Threat, with a significant payoff to Pharma if successful. This Threat may or not be supported by the evidence.
The conventional PEND, and the associated method of calculating rate of return on investment in higher prices excludes the possibility that more drugs in the future will lead to worse health than would otherwise be possible.
It is possible to have a very high and positive estimate of the conventional rate of rate of return on consumer investment but for this to result in lower health for the population in the future.
Reframing the political economy of new drugs to include the possibility that more additional drugs will reduce the population’s health compared with what would otherwise be the case.
The issues addressed in relation to pharmaco-economics follow.
The strategy of reimbursement has two actions: adoption and displacement.
The endogeneity of the price of new drug; it is the result of negotiation, regulation and bargaining power, not the result of an RCT, a systematic review of the literature nor the adjustment of a charge to become a cost. Prices are not constants.
The IPER, IMER, and IπER, which were developed to facilitate the math, highlight different sources of innovation (including manufacturing innovation) and the appropriation of economic rent from previous innovative NMEs by subsequent NMEs.
The distinction between types of budgets: fixed, constrained, unconstrained and no budget.
The use of game theory to engage with Pharma’s rent-seeking (lobbying).
The idea of a health shadow price that accommodates allocative or technical inefficiency as competition in the institution’s market for health inputs.
PEA, which compares the effect of the strategy of reimbursement on the population with the best alternative strategy, giving a value to the evidence of the counterfactual.
The Reimburser also acknowledges that there are many opportunities for further research. For example, expected value of information methods such as those described in Drummond et al. (2005) and by Eckermann and Willan (2007) could be used to identify and quantify the value of reducing the uncertainty around β c . Conventional expected value of information methods could be adapted to accommodate endogeneity of the new drug price, which is assumed to be exogenous in current models. But is all this enough to ensure that β c will be adopted as the threshold price?
11.3 The Next Problem
The Reimburser is surprised when a chorus of criticism of β c as the decision threshold of choice emanates from, not Pharma and pharma-economists, but the health economic community. She identifies nine arguments against using β c and for using either k or d. She is also provided with a book chapter by a US health economist that suggests a theoretical approach to incorporating the cost of R&D in a CEA (Pauly 2007). Each of these arguments are addressed in the following sections.
11.3.1 Benefits Beyond QALYs
The first criticism is that β c assumes that there is no benefit from a new drug other than the incremental QALYs. There are many cases when a new drug has additional benefits beyond health, for example, it could also improve productivity. Alternatively, the new drug could address “equity” in situations where patients with end-stage cancer have no other treatment options. Finally, characteristics of patients such as disease severity could also be seen to have a value independent of capacity to benefit in terms of health effects.
The Reimburser is so confident in the answer to this question that she does not even ask her Health Economist Adviser. She refers to Chap. 6 and shows that if the objective of the reimbursement process is changed to, for example, “QALY plus other thing”, so should the set of alternative strategies from which the best alternative strategy is selected. If the impact of the drug on factors such as productivity and equity are measurable and valued, then any means of achieving the same outcomes should be assessed. In fact, if the additional non-health benefits of the new drug are also valued, then it is possible that the most cost-effective alternative strategy results in a shadow price lower than β c , a “health+” shadow price; a counterintuitive but plausible result. [See Pekarsky (2012, Appendix 10) for an example of this situation.]
11.3.2 But No One Will Implement the Best Alternative Strategy
The second criticism is: what if β c is applied, the new drug is rejected because f > β c , but then the best alternative strategy is not adopted? The Reimburser is confused. What does this mean? Is this concern simply a justification for the decision to reimburse the new drug at a higher threshold? This justification is underpinned by the following or similar logic.
If we reject the drug at the offer IPER because f > β c even though d > f, no one will actually perform the reallocation to the best alternative strategy. Therefore, we might as well just reimburse at f because at least the population’s health will increase.
In this case the appropriate response is to explicate and solve the following paradox:
Why would an Institution not adopt an alternative more effective strategy, but be willing to reimburse the new drug instead, even if this action foregoes the more cost-effective opportunity?
Perhaps the costs of reducing allocative efficiency are too high? If there are costs to improving allocative efficiency, then these should be incorporated into the estimate of the β c by redefining the set of alternative strategies. However, in this case the costs of the uptake of significant new drugs should also be included in the IPER, for example, the ongoing costs of prescriber education programs. Any reason that an Institution can give to not adopt the more effective strategy should be analysed and, where appropriate, accommodated in the estimate of β c . What if all of the possible explanations are exhausted and a reluctance to implement the best alternative strategy remains? Then it is a question of “finding the market or institutional failure”, that is, working out why the Institution will not implement the better strategy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


