Morphogenesis is accomplished in the developing organism by the coordinated interplay of the mechanisms introduced in this section. In some contexts, morphogenesis is used as a general term to describe all of development, but this is formally incorrect because morphogenesis has to be coupled to the process of growth discussed here to generate a normally shaped and functioning tissue or organ.
Human Embryogenesis
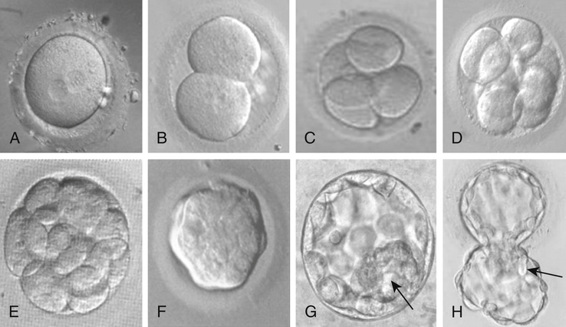
This description of human development begins where Chapter 2 ends, with fertilization. After fertilization, the embryo undergoes a series of cell divisions without overall growth, termed cleavage. The single fertilized egg undergoes four divisions to yield the 16-cell morula by day 4 (Fig. 14-9). At day 5, the embryo transitions to become a blastocyst, in which cells that give rise to the placenta form a wall, inside of which the cells that will make the embryo itself aggregate to one side into what is referred to as the inner cell mass. This is the point at which the embryo acquires its first obvious manifestation of polarity, an axis of asymmetry that divides the inner cell mass (most of which goes on to form the mature organism) from the embryonic tissues that will go on to form the chorion, an extraembryonic tissue (e.g., placenta) (Fig. 14-10). The inner cell mass then separates again into the epiblast, which will make the embryo proper, and the hypoblast, which will form the amniotic membrane.
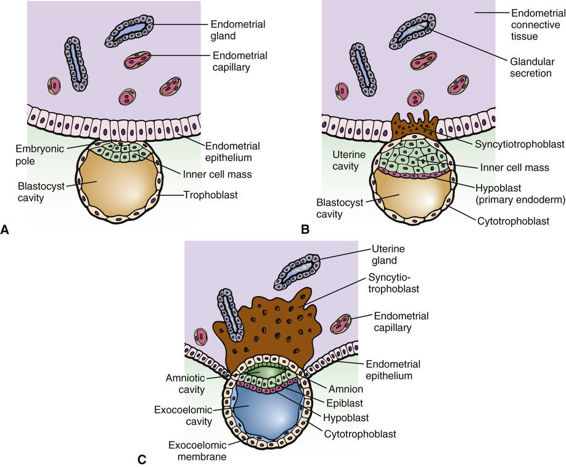
The embryo implants in the endometrial wall of the uterus in the interval between days 7 and 12 after fertilization. After implantation, gastrulation occurs, in which cells rearrange themselves into a structure consisting of three cellular compartments, termed the germ layers, comprising the ectoderm, mesoderm, and endoderm. The three germ layers give rise to different structures. The endodermal lineage forms the central visceral core of the organism. This includes the cells lining the main gut cavity, the airways of the respiratory system, and other similar structures. The mesodermal lineage gives rise to kidneys, heart, vasculature, and structural or supportive functions in the organism. Bone and muscle are nearly exclusively mesodermal and have the two main functions of structure (physical support) and providing the necessary physical and nutritive support of the hematopoietic system. The ectoderm gives rise to the central and peripheral nervous systems and the skin. During the complicated movements that occur in gastrulation, the embryo also establishes the major axes of the final body plan: anterior-posterior (cranial-caudal), dorsal-ventral (back-front), and left-right axes, which are discussed later.
The next major stages of development involve the initiation of the nervous system, establishment of the basic body plan, and then organogenesis, which occupies weeks 4 to 8. The position and basic structures of all of the organs are now established, and the cellular components necessary for their full development are now in place. It is during this phase of embryonic development that neural tube defects occur, as we explore next.
Neural Tube Defects
Neural tube defects (NTDs) are among the most common and devastating birth defects. Anencephaly and spina bifida are NTDs that frequently occur together in families and are considered to have a common pathogenesis. In anencephaly, the forebrain, overlying meninges, vault of the skull, and skin are all absent. Many infants with anencephaly are stillborn, and those born alive survive a few hours at most. Approximately two thirds of affected infants are female. In spina bifida, there is failure of fusion of the arches of the vertebrae, typically in the lumbar region. There are varying degrees of severity, ranging from spina bifida occulta, in which the defect is in the bony arch only, to spina bifida aperta, in which a bone defect is also associated with meningocele (protrusion of meninges) or meningomyelocele (protrusion of neural elements as well as meninges through the defect; see Fig. 17-3).
As a group, NTDs are a leading cause of stillbirth, death in early infancy, and handicap in surviving children. Their incidence at birth is variable, ranging from almost 1% in Ireland to 0.2% or less in the United States. The frequency also appears to vary with social factors and season of birth and oscillates widely over time (with a marked decrease in recent years; see later discussion).
A small proportion of NTDs have known specific causes, for example, amniotic bands (see Fig. 14-3), some single-gene defects with pleiotropic expression, some chromosomal disorders, and some teratogens. Most NTDs, however, are isolated defects of unknown cause.
Maternal Folic Acid Deficiency and Neural Tube Defects.
NTDs were long believed to follow a multifactorial inheritance pattern determined by multiple genetic and environmental factors, as introduced generally in Chapter 8. It was therefore a stunning discovery to find that the single greatest factor in causing NTDs is a vitamin deficiency. The risk for NTDs was found to be inversely correlated with maternal serum folic acid levels during pregnancy, with a threshold of 200 µg/L, below which the risk for NTD becomes significant. Along with reduced blood folate levels, elevated homocysteine levels were also seen in the mothers of children with NTDs, suggesting that a biochemical abnormality was present at the step of recycling of tetrahydrofolate to methylate homocysteine to methionine (see Fig. 12-8). Folic acid levels are strongly influenced by dietary intake and can become depressed during pregnancy even with a typical intake of approximately 230 µg/day. The impact of folic acid deficiency is exacerbated by a genetic variant of the enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR), caused by a common missense mutation that makes the enzyme less stable than normal. Instability of this enzyme hinders the recycling of tetrahydrofolate and interferes with the methylation of homocysteine to methionine.
The mutant allele is so common in many populations that between 5% and 15% of the population is homozygous for the variant. In studies of infants with NTDs and their mothers, it was found that mothers of infants with NTDs were twice as likely as controls to be homozygous for the mutant allele encoding the unstable enzyme. How this enzyme defect contributes to NTDs and whether the abnormality is a direct result of elevated homocysteine levels, depressed methionine levels, or some other metabolic derangement remain undefined.
Prevention of Neural Tube Defects.
There are two approaches to preventing NTDs. The first is to educate women to supplement their diets with folic acid 1 month before conception and continuing for 2 months after conception during the period when the neural tube forms. Dietary supplementation with 400 to 800 µg of folic acid per day for women who plan their pregnancies has been shown to reduce the incidence of NTDs by more than 75%. Much active discussion is ongoing as to whether the entire food supply should be supplemented with folic acid as a public health measure to avoid the problem of women failing to supplement their diets individually during pregnancy.
The second approach is to apply prenatal screening for all pregnancies and offer prenatal diagnosis to high-risk pregnancies. Prenatal diagnosis of anencephaly and most cases of open spina bifida relies on detecting excessive levels of alpha-fetoprotein (AFP) and other fetal substances in the amniotic fluid and by ultrasonographic scanning, as we shall discuss further in Chapter 17. However, less than 5% of all patients with NTDs are born to women with previous affected children. For this reason, screening of all pregnant women for NTDs by measurements of AFP and other fetal substances in maternal serum is now widespread. Thus we can anticipate that a combination of preventive folic acid therapy and maternal AFP screening will provide major public health benefits by drastically reducing the incidence of NTDs.
Human Fetal Development
The embryonic phase of development occupies the first 2 months of pregnancy and is followed by the fetal phase of development, which is concerned primarily with the maturation and further differentiation of the components of the organs. For some organ systems, development does not cease at birth. For example, the brain undergoes substantial postnatal development, and limbs undergo epiphyseal growth and ultimately closure after puberty.
The Germ Cell: Transmitting Genetic Information
In addition to growth and differentiation of somatic tissues, the organism must also specify which cells will go on to become the gametes of the mature adult. The germ cell compartment serves this purpose. As described in Chapter 2, cells in the germ cell compartment become committed to undergoing gametogenesis and meiosis in order that the species can pass on its genetic complement and facilitate the recombination and random assortment of chromosomes. In addition, the sex-specific epigenetic imprint that certain genes require must be reset within the germ cell compartment (see Chapters 3, 6, and 7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


