Chapter 7 Composition of essential oils and other materials
This chapter will look at the composition of a number of popular aromatherapy materials. There is a lot of published data describing chemical compositions. This can be found in varying degrees of complexity in the aromatherapy books and, additionally, from a number of bodies such as ISO (International Organization for Standardization), RIFM (Research Institute for Fragrance Materials), IFRA (International Fragrance Association), AFNOR (Association Française de Normalisation) and the BP (British Pharmacopoeia).
QUALITY CONTROL
The composition of commercially available essential oils cannot be artificially controlled as they are products of natural origin. However, when the essential oils are analyzed their composition should be within a range of acceptable values. If these values are not met, the oil should be rejected. This ensures a standard in the product supplied so that it should perform in a consistent manner when used therapeutically. QC is necessary to protect supplier, practitioner and client from any unforeseen effects of aromatherapy performed with oils that might present risks due to unknown properties.
BACKGROUND TO COMPOSITION
It should be remembered that the analysis for any substance, including essential oils, can only be true for that substance at the time the tests were performed. The composition of oils can subsequently change with handling and storage, so by the time it reaches you, or by the time you use it, it may have a very different composition.
ORGANIC OILS
Strict regulations, known as standards, define their practice. The International Federation of Organic Agricultural Movements (IFOAM) lays down EU standards. In the UK the government sets the standards, which also meet the European and international standards set by their certifying bodies. Each one has its own symbol and EU code number. The main certifying body in the UK is the Soil Association, its UK code is 5 and its organic symbol appears on approximately 70% of organically produced food in the country. Others include The Organic Farmers and Growers Ltd (OF & G) UK code 2, The Scottish Organic Producers Association (SOPA) – UK3, The Irish Organic Farmers and Growers (IOFGA) – UK7 and the Quality Welsh Food Certification – UK13. Figure 7.1 shows two examples of organic organization logos.
Symbols
Each EU country also has its own organic certification authority and code. For food and products imported from outside Europe the situation is more complex.
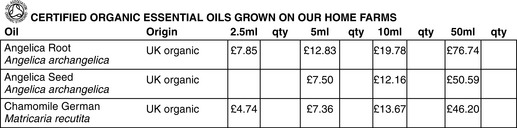
When you buy an organic oil there will not be an accompanying certificate. Currently essential oils and hydrosols do not come under the EU Directive for organic agriculture as it only covers food. It is the grower that has the certification and their literature should show the symbol of their certifying body. See the price list (Fig. 7.2) from a British company that grows and produces essential oils and products. The producer, in this case the farmer, has a UK Soil Association Licence number, which in this case is G4918 and they also have a licence H7677 which means they have been inspected and satisfy the Soil Association standards for organic health and safety products.
You will usually pay more for an authentic organic essential oil as the cost reflects its production. Typically, at the time of writing, an organic lavender (Lavandula angustifolia) would cost up to 81% more than the standard one. However price is not always a reliable guide and as the number of organic harvests expands prices are dropping. Also with organic wild harvested plants the prices are comparable. However, many aromatherapists are willing to pay a premium as they feel they are supporting a more sustainable and ecologically balanced environment for the future.
SPECIAL PROPERTIES OF ESSENTIAL OILS: SYNERGY AND QUENCHING
This can be illustrated by the following considerations:
Synergy
Examples of synergism include the following.
Quenching
The term quenching is used when one component will suppress the harmful effects of another. A knowledge of this is used in safe applications of essential oils. The following are typical examples.
CHEMICAL PURITY AND STANDARD SAMPLES
Definitions and regulations relevant to composition and purity
It is useful at this point to redefine and clarify the types of materials used in aromatherapy. The AOC – Aromatherapy Organizations Council, which represents 75% of UK oil suppliers – has produced the following definitions to assist Trading Standards officers.
REGULATORY AND ADVISORY BODIES
The International Organization for Standardization
This is a worldwide federation of national standards bodies drawn from 130 countries. It was established in 1974 and is based in Geneva, as a nongovernmental body with a mission to promote worldwide standardization. It aims to facilitate international exchange of goods and services and to develop intellectual, scientific, technological and economic activity. Its work has resulted in international agreements, which are published as International Standards that cover a wide range of technologies and services. Technical work is carried out in 218 technical committees (TCs) and the one covering essential oils is TC 54. Its activities cover a wide range of procedures such as packaging, conditioning and storage (ISO/TR210:1999), sampling (ISO212:1973), determination of optical rotation (ISO592:1998) and composition of oils. The composition of each essential oil will also have an individual reference and identification; e.g. oil of rosemary (Rosmarinus officinalis Linnaeus) is ISO1342:1988, and oil of basil, methyl chavicol type (Ocimum basilicum Linnaeus), is ISO11043:1998. These specifications were largely set up for the food and cosmetics industry to give specifications to ensure similarity of products.
British Pharmacopoeia
National pharmacopoeias were developed for the practice of pharmacy so that it should conform to standards laid down in the official pharmacopoeia of the relevant country. The British Pharmacopoeia (BP) is a government-approved list giving details of the manufacture, dosage, uses and characteristics of drugs. It is compiled by experts in pharmacy and pharmacology. The World Health Organization (WHO) has issued the Pharmacopoeia Internationalis in an attempt to standardize drug preparations throughout the world.
CHEMICAL COMPOSITION OF ESSENTIAL OILS
An aromatherapy-grade essential oil may contain up to 400 components. However, a typical GC analysis will detect over 200 but only chemically identify up to 15 of the major components. These components may not include all those compounds that play a role in the action of the essential oil. The analysis is very useful, though, for identifying oils and detecting any adulteration.
Classification and species
| Family: | Labiatae or Lamiaceae |
| Genus: | Lavandula |
| Species: | angustifolia (true lavender) |
| spica (spike lavender) | |
| hybrida (lavandin). |
Developments in botanical classification
The botanical classification that we currently use is attributed to the Swedish botanist Carl Linnaeus. He developed the binomial (two-name) system as a simplification of previously used long Latin names, e.g. Wild Briar Rose was Rosa sylvestris alba cum rubore, folio glabro or Rosa sylvestris indora seu canina pre-Linnaeus – he changed it to Rosa canina, which we abbreviate further to R. canina. His groupings of plants were based on the arrangements and numbers of the stamens (male sex organs) and pistils (female sex organs). This could lead to a classification that did not always seem natural. The roses were associated with the genus Saxifraga (commonly known as the saxifrages) that are a wide range of perennials including alpines. However in spite of its limitations it was simple to use. With our understanding of DNA (deoxyribonucleic acid), which is the genetic material of most living organisms, and the progress in genome sequencing (the genes contained in the chromosomes of the organism) plant classification using this is being discussed. It shows an understanding of plants grouped according to their natural and evolutionary relationships. Under this new system roses are in the genus Urtica that contains nettles. However, classical taxonomy (which is the theory, practice and rules of classification) will continue to rely on morphology (structure) in plants as well as molecular data systems.
EXAMINATION OF ESSENTIAL OILS AND OTHER MATERIALS
| Family | Essence/Essential oil |
| Lamiaceae (Labiatae) | 1. The Lavenders |
| 2. Clary Sage (Salvia sclarea) | |
| 3. Marjoram (Origanum marjorana) | |
| 4. Rosemary (Rosemarinus officianalis) | |
| 5. Thyme (Thymus vulgaris) | |
| 6. Peppermint (Mentha piperita) | |
| 7. Basil (Ocimum basilicum) | |
| 8. Patchouli (Pogostemon cablin) | |
| Rutaceae | 9. Neroli (Citrus aurantium) |
| 10. Petitgrain (Citrus aurantium) | |
| 11. Bitter orange (Citrus aurantium) | |
| 12. Sweet orange (Citrus sinensis) | |
| 13. Bergamot (Citrus bergaia) | |
| 14. Lemon (Citrus limon) | |
| 15. Mandarin (Citrus nobilis) | |
| 16. Grapefruit (Citrus paradisi) | |
| Graminae (Poaceae) | 17. Lemongrass (Cymbopogon citratus) |
| 18. Vetivert (Vetiveria zizanioides) | |
| Asteraceae (Compositae) | 19. Chamomiles |
| Myrtaceae | 20. The Eucalyptuses |
| 21. Tea Tree (Melaleuca alternifolia) | |
| Geraniaceae | 22. Geranium (Pelargonium graveolens) |
| Piperaceae | 23. Black Pepper (Piper nigrum) |
| Apiaceae (Umbelliferae) | 24. Fennel (Foeniculum vulgare) |
| Rosaceae | 25. Roses |
| Oleaceae | 26. Jasmine (Jasminium grandiflorum) |
| Annonaceae | 27. Ylang-Ylang (Cananga odorata) |
| Santalaceae | 28. Sandalwoods |
| Burseraceae | 29. Frankincense (Boswellia sacra) |
| 30. Myrrh (Commiphora myrrha) | |
| Styracaceae | 31. Benzoin (Styrax benzoin) |
| Zingiberaceae | 32. Ginger (Zingiber officinale) |
| Pinaceae | 33. Cedarwoods |
| Cupressaceae | 34. Cypress (Cupressus sempervirens) |
| 35. Juniper (Juniperus communis) |
Uses and actions of essential oils
The uses and actions of essential oils are briefly mentioned in the descriptions of the essential oils and are of paramount importance to the aromatherapist. There is a vast amount of published data, some the results of scientific work but much based in traditional folklore and anecdotal accounts. Information is often contradictory and many different oils appear to have the same properties. Information is beyond the scope of this book to go into clinical usage. A chemical or molecular approach is important for understanding properties and safe applications. However, aromatherapy is still an essentially holistic therapy and also needs experience, intuition and partnership between therapist and client.
POPULAR ESSENCES
Lamiaceae (Labiatae)
1 The lavenders (true, lavandin and spike) (Figs. 7.3, 7.4, 7.5)
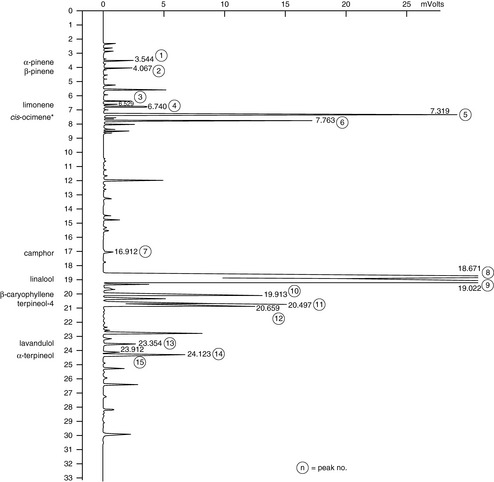
Figure 7.3 Lavender. A good-quality French lavender, true lavender Lavandula angustifolia. This shows a high linalyl acetate content (33.29%) and low camphor content (0.21%). This oil would meet the ISO standards.
Courtesy of Jenny Warden, Traceability.
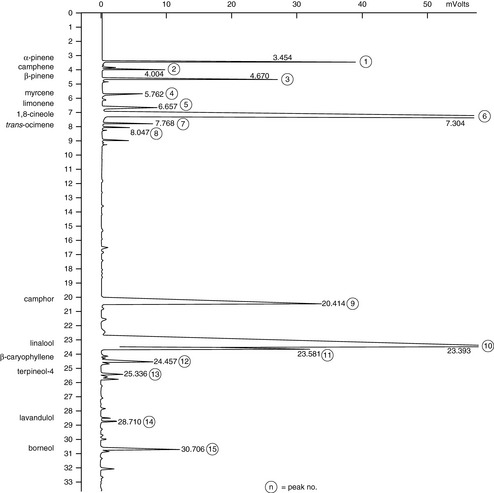
Figure 7.4 Lavender. This shows the GC analysis for spike lavender (Lavandula latifolia). The camphor level is high and that of linalyl acetate is low.
Courtesy of Jenny Warden, Traceability.
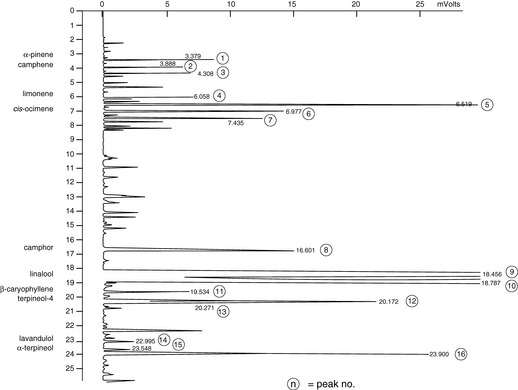
Figure 7.5 Lavender. Analysis of Lavandula hybrida, showing a composition that is intermediate between the true lavender and spike lavender.
Courtesy of Jenny Warden, Traceability.
Lavender is a long-established essential oil, with a legendary folk tradition. The use of ‘just lavender’ is very misleading. The true lavender is from Lavandula angustifolia (also called Lavandula officinalis, Lavandula vera). This is divided into other subspecies Lavandula delphinensis and Lavandula fragrans. Lavandula angustifolia Miller is a lavender grown in France and is the only one recognized in the French Pharmacopoeia. Combined with the existence of many chemotypes, this gives an indication of the many possibilities for variation in the oil.
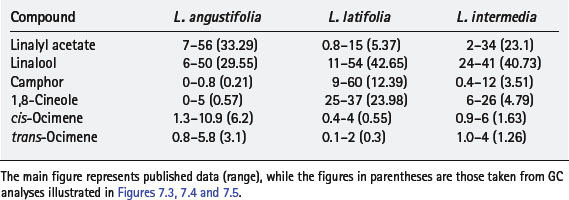
Chemically, all forms contain linalyl acetate, linalool and 1,8-cineole, along with many other compounds. Further analysis of each type reveals their differences in amounts of chemical components. The situation is illustrated by comparing published data for principal constituents and then seeing how these are reinforced by an actual GC chromatogram. This is shown in Table 7.1; the main figure is the published data while figures in brackets are those taken from the GC analysis of actual oil samples (cis– and trans-ocimene are minor hydrocarbon components, but are included as they are often used as markers for the authenticity of lavender oils). In all cases the amounts of compounds in the hybrid (Lavandula intermedia) are in between those of the true (Lavandula angustifolia) and the spike (Lavandula latifolia).
The true lavender (Lavandula officinalis) shown in the chromatogram is high in linalyl acetate, conforming to the ISO standard composition range of 25–45% and linalool ISO standard of 25–38%. True lavenders also may have between 5% and 30% lavandulyl acetate; the GC for this sample shows quite a low value of 3.55%. Also characteristic of true lavender, the amounts of camphor and the oxide 1,8-cineole are low, but are increased in the other species. High ester and alcohol content makes this a desirable aromatherapy choice as it is gentle with no known contraindications.
2 Clary Sage (Salvia sclarea)
Several different chemotypes occur according to their geographical region of growth. They are mainly Mediterranean regions but also USA, Russia, Morocco and France. The essential oil has a heavy fragrance with herbal and nutty tones and pale green/yellow to clear colour. The odour can be attributed to the major component the ester linalyl acetate that can be present as up to 75% of the total composition. Also present alcohol linalool (up to 26%), terpenes β-caryophyllene (up to 3%), germacrene (up to 4%), neryl acetate (up to 1.7%). Box 7.1 shows a certificate of analysis which gives a more comprehensive insight to the chemical composition. Therapeutic applications are linked to antidepressive, spirit lifting and creative stimulating actions on the mind. Reputed to act as an antispasmodic and emmenagogue, and promoting the female hormone oestrogen it is used to ease PMS (pre-menstrual syndrome) and encourage and ease labour. Also used for respiratory conditions including asthma and for a range of skin conditions associated with greasy complexions such as acne, boils and dandruff. Although considered to be nontoxic, nonirritant and nonsensitizing it should be avoided during pregnancy.
Box 7.1 Certificate of analysis for Clary Sage (Salvia sclarea)
Courtesy of Jane Collins, Phytobotanica
CERTIFICATE OF ESSENTIAL OIL ANALYSIS 2007
ESSENTIAL OIL OF CLARY SAGE (Salvia sclarea)
METHOD OF EXTRACTION – Steam Distillation
| Peak No | Compound | % Composition |
|---|---|---|
| 1 | Myrcene | 0.86 |
| 2 | Limonene | 0.51 |
| 3 | E-Ocimene | 0.35 |
| 4 | α-copaene | 0.75 |
| 5 | linalol | 9.90 |
| 6 | linalyl-acetate | 72.20 |
| 7 | β-caryophyllene | 2.11 |
| 8 | germacrene D | 2.57 |
| 9 | α-terpineol | 1.38 |
| 10 | neryl acetate | 1.17 |
| 11 | alkene | 0.34 |
| 12 | geranyl acetate | 2.06 |
| 13 | nerol | 0.52 |
| 14 | geraniol | 1.33 |
| 15 | caryophyllene oxide | 1.17 |
| 16 | spathulenol | 0.13 |
| 17 | alcohol | 0.68 |
| 18 | alcohol | 1.97 |
3 Marjoram (Origanum marjorana)
Sweet marjoram is a pale yellow oil with a warm, camphoraceous and spicy odour. Its main components are alcohols terpinen-1-ol-4 (14–20%), thujan-4-ol (4–13%), linalool (2–10%), α–terpineol (7–27%), hydrocarbon monoterpenes sabinene (2–10%) β-myrcene (1–9%), β-terpinolene (1–7%), α-pinene (1–5%), α–terpinene (6–8%), ester geranyl acetate (1–7%) and aldehyde citral (4–6%). Box 7.2 shows a GC analysis report. A versatile oil with many claimed therapeutic properties. Often called a comforting oil. Applied to the mind in situations of stress and grief where it calms and relaxes. For the body it is considered warming, analgesic, and antispasmodic suitable for muscle and joint pains. Also acting on the respiratory system for asthma and bronchitis, on the digestive system as a carmative relieving cholics and constipation and in skincare for bruising and chilblains. Considered a safe nontoxic, nonirritating and nonsensitizing essential oil but should be avoided during pregnancy.
Box 7.2 Certificate of analysis for Sweet Marjoram (Origanum marjorana)
Courtesy of Geoff Lyth, Quinessence Aromatherapy Ltd
GAS CHROMATOGRAPHY REPORT
| Product Identification | |
|---|---|
| Product Name : | Marjoram Sweet Oil |
| Botanical Name : | Origanum marjorana |
| Country of Origin : | Egypt |
| Agricultural Method : | Organic |
| Product Code : | E592 |
| Batch Number : | 764 |
| Principal Constituents | Constituent % |
|---|---|
| α-Thuyene : | 0.58 |
| α-Pinene : | 0.77 |
| Sabinene : | 7.55 |
| Myrcene : | 2.05 |
| α-Phellandrene : | 0.56 |
| α-Terpinene : | 8.00 |
| Limonene : | 1.04 |
| 1,8 Cineole : | 1.95 |
| β-Phellandrene : | trace |
| γ-Terpinene : | 12.80 |
| Terpinolene : | 3.00 |
| trans-Thuyanol-4 : | 3.95 |
| Linalool : | 1.23 |
| Linalyl acetate : | 2.35 |
| cis-Thuyanol-4 : | 17.95 |
| Terpinene-4-ol : | 22.55 |
| α-Terpineol : | 3.17 |
| β-Caryophyllene : | 2.05 |
| Neryl acetate : | 1.27 |
4 Rosemary (Rosmarinus officinalis)
Rosemary oils are derived from one main species with well-documented cultivars that show variations in chemical composition due to the climate they are grown in.
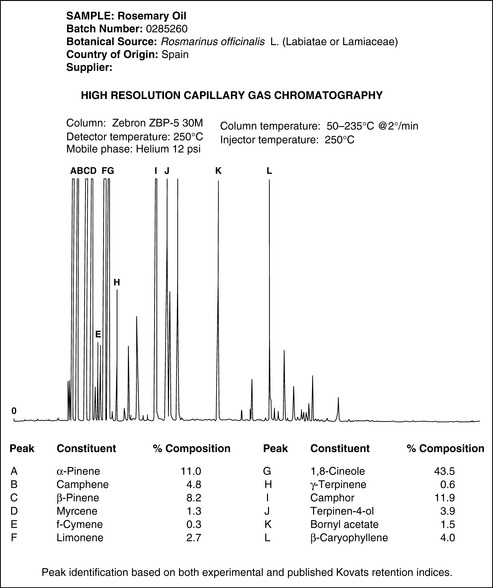
Figure 7.6 Typical supplier’s GC analysis data. Rosemary. Analysis of Rosmarinus officinalis.
Courtesy of Jasbir Chana, Phoenix Natural Products Ltd.
Typically listed chemical components are:
| Terpenes: | camphene, pinene, limonene, myrcene |
| Sesquiterpenes: | caryophyllene, humulene |
| Alcohols: | borneol, linalool, terpineol |
| Ketones: | camphor, thujone, verbenone |
| Aldehyde: | cuminic aldehyde |
| Esters: | bornyl acetate, fenchyl acetate |
| Oxide: | 1,8-cineole |
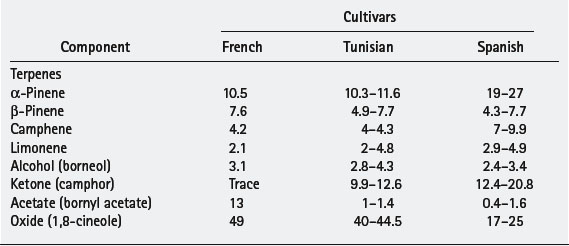
There are three principal chemotypes: verbenone, 1,8-cineole and camphor-borneol. These are examples of variation due to the climate they are grown in and are also called cultivars. The names of the cultivars are not Latinized and appear after the species name, often within quotation marks. For example, Lavandula angustifolia ‘Maillette’ is a type of lavender named after its originator. For rosemary the cultivars are named after their country of origin. As a consequence of this, the verbenone is also called French, the 1,8-cineole is called Tunisian and the camphor-borneol is called Spanish. A comparison of these in terms of their amounts of main components is shown in Table 7.2.
Rosmarinus officinalis essential oil is usually regarded as nontoxic, nonsensitizing and nonirritant when used in sufficient dilution. It may cause dermatitis in hypersensitive individuals and there is some evidence to suggest it should not be used during pregnancy, by epileptics, or by those with high blood pressure. A material safety data sheet from an oil supplier (Box 7.3) shows the type of information available for a Spanish Rosmarinus officinalis. The sample conforms to the data in the table for the Spanish but also has compounds that would fit the ranges given for Tunisian.
Box 7.3 A typical supplier’s data sheet: Rosemary
Courtesy of Phoenix Natural Products Ltd
MATERIAL SAFETY DATA SHEET
ACCORDING TO EC LEGISLATION 91/155/E EC
Date created: June 1998 Date revised: June 1998
Figure 5.6 also shows an IR spectral analysis of Rosmarinus officinalis.
5 Thyme (Thymus vulgaris)
A ‘typical’ analysis of major components of these contrasting chemotypes for T. vulgaris shows this difference (Table 7.3). Typical analysis is again difficult to define, so ‘representative’ analysis might be a better description.
Table 7.3 A representative analysis (%) of phenolic and alcohol chemotypes (CT) of Thymus vulgaris
| Compound | Red-phenolic CT | Sweet-alcohol CT |
|---|---|---|
| Thymol | 30–48 | 2% |
| Carvacrol | 0.5–5.5 | |
| Linalool | 0 | 30–80% linalool CT |
| Geraniol | 0 | 30–80% or geraniol CT |
| Geranyl acetate | 0 | Up to 50% |
| p-Cymene | 18.5–21.4 | 0 |
| 1,8-Cineole | 3.6–15.3 | 0 |
| β-Caryophyllene | 1.3–7.8 | 4 |
| α-Pinene | 0.5–5.7 | 0 |
| Terpinolene | 1.8–5.6 | 0 |
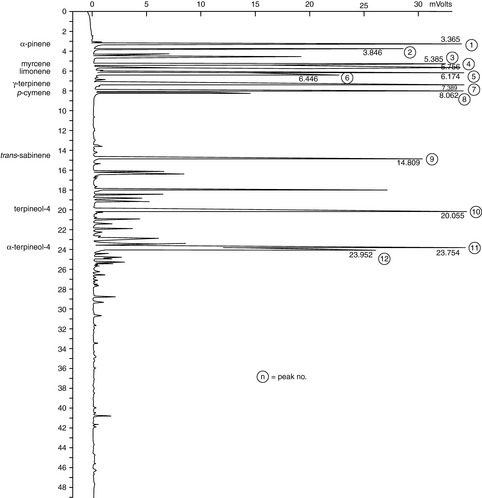
The GC analysis (Fig. 7.7) shows a commercial white thyme with a high percentage of the phenols thymol (55.8%) and carvacrol (2.07%). This contrasts with that of sweet thyme (Fig. 7.8) with no phenolic compounds present and alcohols terpineol-4 (13%), α-terpineol (12.37%) and borneol (5.95%) making up a total of 31.34% for this particular sample.
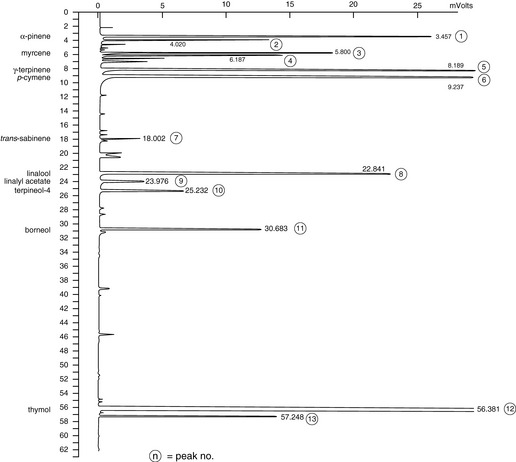
Figure 7.7 Thyme. A typical white Spanish thyme, showing the presence of thymol. Thymol has a retention time of 56.381 min and makes up 55.58% of the oil in this GC analysis of this particular sample.
Courtesy of Jenny Warden, Traceability.
6 Peppermint (Mentha piperita)
There are numerous species of mint including peppermint, Mentha piperita, spearmint, Mentha spicata, and cornmint, Mentha arvensis. Mentha piperita is actually a hybrid species bred from spearmint Mentha spicata and watermint Mentha aquatica. They all contain subspecies and chemotypes. Mints have a long tradition of culinary, fragrance, cosmetic and therapeutic applications.
Typical composition would be: menthol (27–51%), menthone (13–32%), isomenthone (2–10%), 1,8-cineole (5–14%), methyl acetate (2–4%), methofuran (2–12%), limonene (0.5–6%), pinenes (1.5–4%), germacrene (2.1–4.3%) and pulegone (0.1–1%). Box 7.4 shows a GC analysis for Mentha piperita.
Box 7.4 Analysis of Peppermint (Mentha piperita)
Courtesy of Geoff Lyth, Quinessence Aromatherapy Ltd
GAS CHROMATOGRAPHY REPORT
| Product Identification | |
|---|---|
| Product Name : | Peppermint (Mitcham) Oil |
| Botanical Name : | Mentha piperita |
| Country of Origin : | United Kingdom |
| Agricultural Method : | Organic |
| Product Code: | E622 |
| Batch Number : | 692 |
| Principal Constituents | Constituent % |
|---|---|
| α-Pinene | 0.66 |
| β-Pinene | 0.98 |
| Sabinene | 0.52 |
| Limonene | 1.44 |
| 1,8 Cineole | 5.41 |
| z-Ocimene | 0.64 |
| Menthone | 14.13 |
| cis-Sabinene-hydrate | 1.70 |
| Benzofuran, 4,5,6,7 etc | 5.97 |
| iso-Menthone | 2.67 |
| Menthyl acetate | 6.30 |
| Cyclohexanol | 5.65 |
| Menthol | 50.98 |
| Germacrene D | 1.44 |
| 2-Cyclohexan-1-one, etc | 0.70 |
| Veridifloral | 0.81 |
Therapeutic uses are widespread as peppermint has a skin toning effect and is most effective for digestive disorders such as indigestion and flatulence; it stimulates cardiovascular and lymphatic systems, works on ligaments for joint and muscle pain and has local antiseptic properties.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree