Elevated Plus Maze (Neophobia) Test
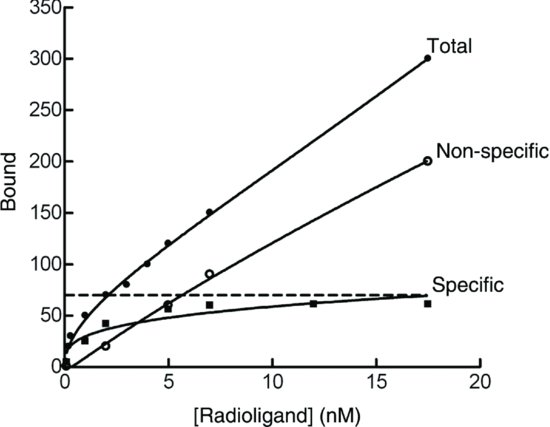
The test apparatus consists of two open arms (45 cm×15 cm) and two enclosed arms at 180° to each other. The entire maze was made out of 5 mm thick black perspex and raised 50 cm above the ground. Each rat was given a 5 min trial, and the time spent in the open arms and total (open + enclosed) number of arm entries were recorded (Table 9.2). Injections were administered as for the Geller–Seifter conflict test above.
Nociception (Hot Plate) and Locomotor Activity Tests
To check for false positives in the conflict and neophobia tests, the effects of the novel drugs on nociception (using morphine as a positive control) and spontaneous locomotor activity (LMA) (using amphetamine as positive control) were assessed. To test for any anti-nociceptive effects, rats were placed on a metal plate maintained at a temperature of 56°C and the time taken to lift their forepaws off the plate (reaction time) was recorded. To measure LMA, rats were placed in a darkened box (45×30×30 cm) with parallel rows of photocells placed 4 cm above the flaw of the box. Activity counts (photocell beam interruptions) were recorded over a 5 min period (Table 9.3). All drugs were administered 30 min (s.c.) before the experiment, as for the other tests.
Questions
Anxiolytic Drug Data Interpretation
Table 9.2 Elevated plus maze data. Each value represents the median time (s) spent in the two open arms or median total time of arm entries over 5 min, for n = 10 rats.
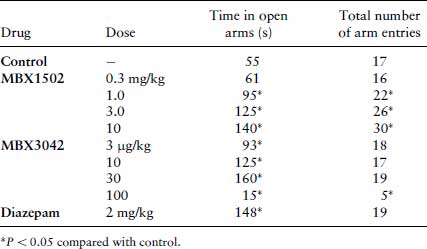
Table 9.3 Nociception and LMA test data. Each value represents the median reaction time (s) or median number of activity counts measured over 5 min for n = 10.
9.2.2 Analysis of Metabolites of 5-hydroxytryptamine
The action of drugs interfering with the metabolism of 5-hydroxytryptamine (5-HT) in the brain are of interest because alterations in 5-HT have been implicated in several altered behavioural conditions, such as affective disorders, anxiety disorders and schizophrenia. The precursor of 5-HT is dietary L-tryptophan that is converted to 5-HT by tryptophan hydroxylase and dopa decarboxylase. 5-HT is degraded to 5-hydroxindoleacetic acid (5-HIAA) by the enzymes monoamine oxidase and aldehyde dehydrogenase. These metabolic products can be separated by chromatography. In the following exercise you are required to analyse and interpret the actions of drugs on 5-HT metabolism.
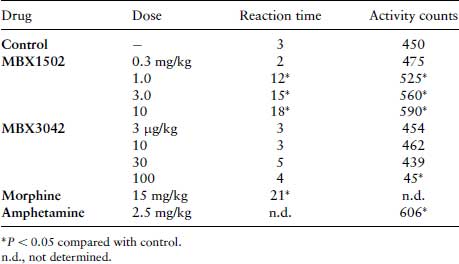
The following data was adapted from Reinhard et al. (2). Three groups of Lister Hooded rats were injected with 75 mg/kg pargyline at 1.5 h, 2.5 mg/kg at 4 h or saline (control) at 1.5 h, before the whole brain was removed and homogenized in 10 volumes of ice-cold 0.2 M HClO4. Following centrifugation, at 10 000×g for 20 min at 4°C, and the supernatant was passed through a 0.45 μm filter to remove particulates before measuring the concentrations of 5-HT and its metabolite 5-HIAA (5-hydroxyindole 3-acetic acid) using HPLC-ECD (high performance liquid chromatography with electrochemical detection), as described below.
Fifty microlitres of aliquots of supernatant (containing 250 μg protein) were injected via a 50×2.1 mm guard column onto a 300×3.9 mm 5 μm C-18 RP (reverse phase) column. The flow rate of the mobile phase (6% v/v methanol in 0.1 M sodium acetate buffer, pH 4.7; filtered and degassed immediately before use) was set to 2.0 mL/min and the potential of the ECD to 0.7 V. Under these conditions, the retention time of 5-HT was 9 min and for 5-HIAA 12 min. A range of standard indoles (0–40 pmol) made up in 0.2 M HClO4 (Table 9.4), was used to quantify 5-HT and 5-HIAA concentrations in the brain samples (Table 9.5).
Table 9.4 ECD responses for 5-HT and 5-HIAA standards.
| ECD response (nA) | ||
| Indole (pmol) | 5-HT | 5-HIAA |
| 0 | 0.00 | 0.00 |
| 5 | 0.55 | 0.40 |
| 10 | 1.15 | 0.85 |
| 20 | 2.25 | 1.65 |
| 30 | 3.40 | 2.50 |
| 40 | 4.50 | 3.30 |
Table 9.5 ECD responses for 5-HT and 5-HIAA in brain samples from three groups of rats. Group A were injected with pargyline (75 mg/kg), group B were injected with reserpine (2.5 mg/kg) and group C were injected with saline only.
9.2.3 Radioligand Binding
Techniques for measuring the direct binding of drugs (usually antagonists, more accurately termed ‘ligands’) to receptors were first introduced in the 1970s. If the simplest model is assumed, then
(9.1) 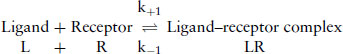
According to the Law of mass action,
(9.2) 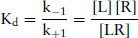
where Kd = equilibrium dissociation constant, and k+1 and k−1 are the association and dissociation and reverse rate constants.
The aim is to determine the affinity of the ligand for the receptor, which is defined as Kd. It is necessary to determine the amount of ligand in the free form [L], and the amount in the bound form [LR]. The earliest techniques involved using a ligand labelled with a radioactive isotope, usually 3H, 14C, 125I, 32P or 35S. The radioactively labelled ligand bound to the receptor to form a ligand–receptor complex. When equilibrium had been reached, the bound ligand was separated from the free ligand, frequently, by filtration through glass-fibre filters or centrifugation.
More recently, other techniques to measure receptor binding have been introduced. Scintillation proximity assays using radioactively labelled ligands can detect bound ligand without separation from the free ligand. Currently, non-radioactive assays based on the detection of fluorescence or chemoluminescence are most commonly used.
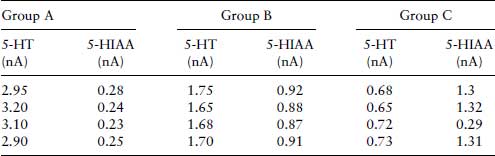
Whichever method is used, some measure of free and bound ligand must be obtained. If the amount of bound ligand found on the filter, [LR], is plotted against the total concentration of ligand pipette into incubation (i.e. [L] + [RL]), a hyperbolic curve that reaches a maximum or plateau, should theoretically be found. In fact, a curve that shows no sign of reaching a plateau results. This is due to a complication of radioactive ligand-binding experiments in that there is a portion of the labelled ligand that binds to low affinity, high-capacity sites other than the receptor, representing binding to the containers, which is termed non-specific binding. This can be measured by carrying out the binding experiment in the presence of saturating concentration of non-radioactive ligand, which displaces radioactive ligand from the receptor sites. The amount of ‘true’ specifically bound ligand is found by first subtracting the non-specific binding from total binding as depicted graphically in Figure 9.1.
Figure 9.1 Total binding, non-specific and specific binding plotted against concentration. Specific binding is the total minus the non-specific binding.