Though not a compendial specification, the PhEur suggests a total aerobic microbial count (TAMC) action limit of 10 CFU/100 mL in its WFI monograph, which agrees with the 1993 FDA Guide to Inspections of High Purity Water Systems. The PhEur and the 1993 FDA guide give a TAMC action limit of 100 CFU/mL for purified water. The USP, in its informational General Chapter <1231> “Water for Pharmaceutical Purposes,” explains that microbial specifications would be inappropriate for a continuously produced material such as bulk pharmaceutical water and for such a validated process. The microbial incubation time lag inherently defeats the real-time production and usage of bulk pharmaceutical water.
The PhEur TAMC action limit statement, in both the purified water and WFI monographs, specifies the use of agar medium S, which is R2A media. Neither the USP nor JP specifies an agar for TAMC of bulk pharmaceutical water, permitting different process validation results to dictate the appropriate medium.
Two guides exist for the frequency and location of bulk pharmaceutical water sampling for monitoring or testing off-line. The 1993 FDA guide also created the generally accepted sampling frequency and sampling locations within a bulk pharmaceutical water distribution system, namely, daily sampling (except during shutdown) from a minimum of one point of use, with all points of use tested weekly. USP General Chapter <1231> states that “sampling frequencies should be based on system validation data and should cover critical areas including unit operation sites. . . . samples should be collected from use points using the same delivery devices, such as hoses, and procedures, such as preliminary hose or outlet flushing, as are employed by production from those use points.”
Pure steam is manufactured such that, when condensed, it satisfies WFI specifications. Guidelines for monitoring pure steam are less well defined to nonexistent compared with those for bulk pharmaceutical waters. Providing it is manufactured within a system that already passes TOC and water conductivity, it is presumed not to deviate significantly from its source pharmaceutical water. In addition, pure steam is inherently self-sterilizing such that TAMC is highly unlikely to provide trends on the steam but only on incorrectly sampled condensate. BET results on pure steam use points or sampling points provides enough assurance against grossly contaminated (by Gram-negative bacteria) draw-off valves.
Water conductivity monitoring/testing may be performed off-line or in-line. In-line conductivity monitoring inherently prevents the progression to stage 2 or stage 3 of the USP or PhEur water conductivity test; however, the distribution system must be designed such that an out-of-specification (OOS) result (based on conductivity versus water temperature) automatically results in either diversion to drain or recirculation into the water generation system. If using the off-line pharmacopeial water conductivity test, stages 2 or 3 must be performed if the previous stage fails. No OOS investigation (per FDA out-of-specification guidance) or stoppage of the conductivity or any multistage compendial test may occur between stages. USP General Chapter <645> requires an electronic calibration of the conductivity meter by replacing the conductivity sensor with a nationally traceable resistance device, for example, Wheatstone Bridge.
The TOC compendial test for bulk pharmaceutical water is harmonized among the USP, PhEur, and JP. The apparatus for measuring TOC, like water conductivity, is either in-line or off-line. The periodic suitability testing of the apparatus is specified as employing both a standard solution of compendial sucrose and a system suitability standard solution of 1,4-Benzoquinone. The diluent/solvent is reagent water having a TOC of not more than 0.10 mg/L.
A monitoring test required for, and applicable only to, WFI and pure steam is the BET. The test, being highly dependent on the particular Limulus amebocyte lysate (LAL) and corresponding test equipment and instruments, is not a candidate for compendial test suitability verification, but must undergo complete method validation for parenteral products. For WFI and pure steam, pharmacopeial test verification of suitability may suffice. The LAL reagent used in the BET has been classified as a biologic, and is licensed by the Center for Biologics Evaluation and Research (CBER). FDA requires that a finished parenteral pharmaceutical manufacturer operating in the US or shipping to the US use an LAL reagent licensed by CBER in all validation, in-process, and end-product LAL tests.
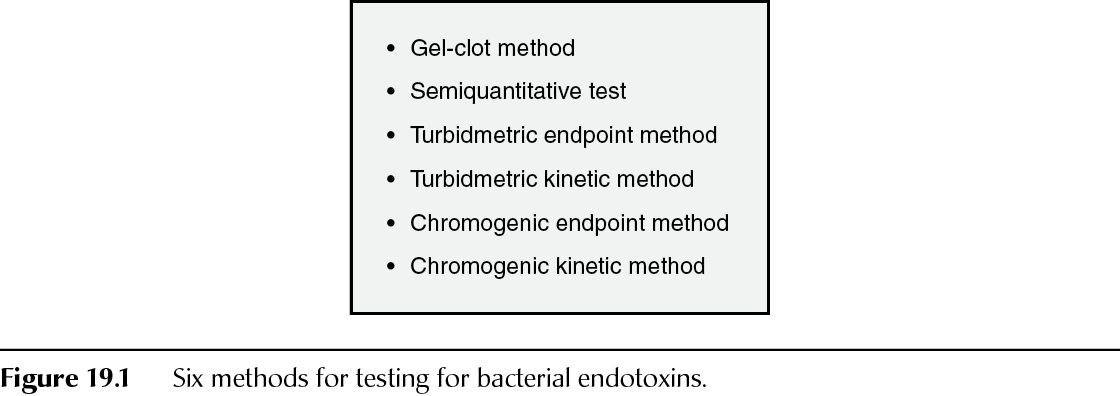
Portions of the BET General Chapter have been harmonized among the three compendia. USP, PhEur, and JP permit and describe six methods (Figure 19.1). All three compendia contain similar wording that “in the event of doubt or dispute, the final decision is made based upon method A [Gel-clot method: limit test] unless otherwise indicated in the monograph [for a specific finished parenteral pharmaceutical].”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


