Commonly Performed Orthopaedic Procedures
Adam Phillip Breceda M.D
Eli Bryk M.D.
Orthopaedic surgical procedures are some of the most commonly performed surgeries worldwide, with musculoskeletal disorders being the second most common reason for patients to seek medical care. It has been estimated that 10 to 30 percent of all primary care provider visits are related to musculoskeletal problems in North America and Europe. In the United States, the direct cost of musculoskeletal medical care and its indirect expenditures in lost wages led to an estimated annual cost of $950 billion, or 77 percent of the gross domestic national product in 2006 (1). This chapter will give a basic description of the indications for surgery of some of the most common orthopaedic surgical procedures, descriptions of those procedures, the specimens that are sent to pathology, along with what the surgeon usually expects from the pathologic analysis. In addition, the recommended processing of each specimen by the pathologist will be described.
Joint Reconstruction Surgery
Total Knee Arthroplasty
Total knee arthroplasty (TKA) usually involves the reconstruction of the knee joint to provide relief of pain from osteoarthritis (OA), also known as degenerative joint disease (DJD) (Fig. 19.1). Approximately 650,000 cases are performed in the United States yearly (2). Osteoarthritis can be classified as primary (wear and tear without any underlying cause) or secondary (due to trauma, dysplasia, osteonecrosis, etc.) (See Table 17.1 Chapter 17).
Indications
The diagnosis of OA is based on history, physical exam, and radiographic imaging. Prior to TKA, nonoperative measures in hopes of slowing disease progression are often initiated. It should be noted, however, that the natural history of OA is disease progression, as there are no disease-modifying agents such as in rheumatoid arthritis. Sometimes, OA progression stabilizes. Nonoperative measures include physical therapy, weight reduction in overweight patients, nonsteroidal anti-inflammatory drugs (NSAIDs) or tramadol, and intra-articular corticosteroid injections (3). The benefits of intra-articular injections of glucosamine products remain controversial. Prior to TKA, especially in younger age group patients, arthroscopy is sometimes performed in hopes of relieving pain and delaying TKA when mechanical meniscal pathology is seen on MRI. In cases of secondary OA caused by anatomic abnormalities, operative correction of those abnormalities is often performed in hopes of halting progression of disease. If no alternative treatment is indicated, the patient is a candidate for TKA.
Procedure
When the patient is taken to the operation theatre, a longitudinal midline incision is often made, with dissection down to the quadriceps tendon, patellar tendon, and patella. An arthrotomy is made to visualize the joint. Surrounding soft tissues such as fat pad, bursa, the anterior cruciate ligament (ACL), and, depending on the implant, the posterior cruciate ligament (PCL) are taken out. Measuring guides are used to determine the proper size of implants to be used. Cutting jigs are used to make bone cuts in the distal femur and proximal tibia. These bone cuts allow proper placement and fixation of the prosthetic implant, while removing the diseased articular surface. Any remaining osteophytes are also removed. Attention is then turned to the patella, where, again, any osteophytes, soft tissue attachments, and fat are removed to demarcate the
undersurface of the patella. The patella is then measured and cut, to allow for placement of the patellar component. Trial components, including femoral, tibial, patellar, and polyethylene components, are inserted, and the knee is tested for stability to varus stress, valgus stress, flexion, and extension. After assuring the knee is stable, the permanent implants are inserted, and fixation is provided with or without polymethylmethacrylate cement.
undersurface of the patella. The patella is then measured and cut, to allow for placement of the patellar component. Trial components, including femoral, tibial, patellar, and polyethylene components, are inserted, and the knee is tested for stability to varus stress, valgus stress, flexion, and extension. After assuring the knee is stable, the permanent implants are inserted, and fixation is provided with or without polymethylmethacrylate cement.
Implants in Total Knee Arthroplasty
The two most common metal alloys in TKA are cobalt chrome and titanium. Cobalt chrome alloys are the most common material used to manufacture femoral components in total knee arthroplasty. The advantage of cobalt chrome alloys is that they have little biological activity and are highly resistant to corrosion. They are also relatively strong and have good surface properties, which make them an excellent choice for bearing surfaces.
Titanium alloys are used to manufacture tibial base plates in modular knee designs. They are not suitable for use as bearing surfaces because of low wear resistance.
Ultra high molecular weight polyethylene is commonly used for tibial liners and patellar components in total knee arthroplasty. This plastic is chemically inert with a low coefficient of friction, which allows efficient articulation.
Pathologic Specimens and Processing
The distal femoral bone cuts, proximal tibial bone cuts, and patellar specimens are sent for pathological inspection after TKA. All other tissues, including cartilaginous components such as the menisci, ligaments, and synovium, should be submitted. Oftentimes, the surgeon’s preoperative and intraoperative diagnoses are confirmed (e.g., osteoarthritis, pathologic fracture, benign or malignant lesion). However, sometimes the pathologic evaluation differs from the surgeon’s observations, such as when a lesion thought to be DJD is rheumatoid arthritis or a benign lesion is actually malignant (4).
The pathologist should process the following:
A sample of synovial tissue to assess the degree of inflammation.
The remaining articular cartilage-bone interface to assess tidemarks and subchondral bone.
A piece of bone marrow to assess hematopoietic status.
Any abnormal gross areas of pathology.
Revision of Total Knee Arthroplasty
Revision of a TKA involves the replacement of any combination of components with new components, and can be limited to exchange
of the polyethylene liner or replacement of all components. Approximately 20,000 such revisions are performed yearly in the United States (2). In this section, we focus on the revision of all components.
of the polyethylene liner or replacement of all components. Approximately 20,000 such revisions are performed yearly in the United States (2). In this section, we focus on the revision of all components.
Indications
Revision TKA has many indications. The most common reasons are patellofemoral maltracking, aseptic failure due to component loosening, poor placement of components leading to joint line abnormalities (imbalanced joint), osteolytic wear, catastrophic wear of components, and periprosthetic fractures or infection.
Procedure
Exposure of the knee is generally approached in the same fashion as a primary TKA. The quadriceps extensor mechanism is subluxated, and an extensive synovectomy is performed to provide exposure of the components. Removal of the tibial polyethylene component, followed by the femoral component and the tibial tray, is a standard approach to component removal. Subsequently, attention is turned to the patellar component. These implants are most often placed in a cemented fashion. The surgeon attempts to disrupt the cement- or component-bone interface. Many tools are used to release the components, including saws, osteotomes, punches, burrs, and even ultrasonic instruments. Because of the tight interface formed between bone and cement or components, a great deal of native bone is often sacrificed during implant removal. The typical approach described above can vary, depending on the difficulty in getting each component out.
As there is bone loss with removal of the primary components, bone defects are usually filled, with the use of bone cement for small defects, and allograft for large defects. Stemmed implants may also be used to overcome loss of significant amounts of bone. The most proximal or distal aspects of bone are prepared for knee implant placement. Modular components, or small units of varying sizes and angles that can be pieced together to form the overall functional hardware, are used to reconstruct the knee. Templates are used initially, as with primary TKA, to ensure proper balance, alignment, and stability. When all components are trialed together and deemed stable, the trial implants are removed and replaced with cemented final components.
Pathologic Specimens and Processing
Bone cuts, primary implants, and other soft tissue are sent to pathology, where they can be evaluated for causes of failure of the components. In septic cases, cultures are obtained from all components with ascertainment of the infecting organism and antibiotic susceptibilities.
Several samples of the soft tissue should be processed to ensure adequate and accurate counts of polymorphonuclear leukocytes in the assessment of possible periprosthetic joint infections (see Chapter 17). Any darkened tissue should be adequately sampled and processed to ensure a thorough assessment of wear debris. Whereas cement and polyethylene debris can lighten the tissue, metal debris can lead to a grayish or blackened appearance. The pathologist should use polarized light microscopy as this will often better detect implant wear debris (see Table 17.5, Chapter 17).
Total Hip Arthroplasty
Similar to TKA, total hip arthroplasty (THA) usually involves the reconstruction of the hip joint to provide relief of pain from OA (Fig. 19.2). About 300,000 such operations are performed each year in the United States (2). Osteoarthritis of the hip may be idiopathic or related to anatomic or developmental disorders, such as developmental dysplasia, coxa profunda, Legg-Calvé-Perthes disease, or slipped capital femoral epiphysis. It is important to consider osteonecrosis, which may result from corticosteroid use, sickle cell disease, systemic lupus erythematosus, alcoholism, or Highly active anti-viral therapy (HAART) treatment of human immunodeficiency virus infection. Other important arthritides such as rheumatoid arthritis can be the rationale for a THA (see Table 17.1, Chapter 17).
Indications
The preoperative diagnosis is made with history, physical exam, and radiographs. Initial management usually begins with nonoperative measures including physical therapy, weight loss, treatment with NSAIDs, and the use of walking aids. When nonoperative modalities have failed to provide pain relief and return of function,
and the patient’s activities of daily living are significantly compromised, the patient is considered for THA.
and the patient’s activities of daily living are significantly compromised, the patient is considered for THA.
Procedure
An anterior, anterolateral, or posterolateral incision is made about the hip and carried down through muscular and fascial layers to expose the hip. The hip is dislocated, and a femoral neck osteotomy is performed. The femoral head is removed. Then the acetabulum is exposed and prepared by reaming until cancellous bone is visualized. Cysts are debrided and sclerotic margins are removed. The acetabular component is measured on the basis of the size of reamer used in preparation. The component is then inserted in anatomic alignment to the acetabulum. The proximal femur is then prepared using broaches or cylindrical reamers. During preparation, the size of the broach is used to determine the size of the stem for the femoral component. The femur is broached until the broach is rotationally stable in the canal of the femur, and the proximal end of the broach lines up with the cut of the femoral neck. Trial components are used to determine stability of the reconstructed hip, using the broach, trial necks, and femoral heads in a modular fashion. The acetabular and femoral components may be inserted in an uncemented, press-fit technique, or cemented. A 2014 study showed that 93 percent of THA constructs are performed cementless in the United States (5). After stability is confirmed, the soft tissues and skin wound are closed, and postoperative management is begun.
Implants in Total Hip Arthroplasty
The femoral stems in THA may be made of titanium, cobalt chrome, stainless steel, or polymer composites. Cemented stems use acrylic bone cement to form a mantle between the stem and the bone; typically, these stems are cobalt chromium. Uncemented stems use shape and surface coatings to stimulate bone to remodel and bond to the implant. Typically, these stems are titanium.
Most THAs in use today use modular components, which consist of different head dimensions and modular neck orientations attached to the femoral stem using a “Morse” taper. The femoral heads are generally made of cobalt chromium or ceramic material.
Modular acetabular components consist of two pieces: a shell and a liner. In cementless acetabular arthroplasty, the shell is generally made of titanium with an outside porous coating, while the inside contains a locking mechanism designed to accept a liner made of ultra high molecular weight polyethylene. One piece, mono-block, acetabular components are typically made of ultra high molecular weight polyethylene, which is then cemented directly into the acetabulum.
Pathologic Specimens and Processing
Specimens sent to pathology include femoral head and neck, synovium, cartilage, and medullary contents. The femoral head can be divided to look for abnormal anatomy such as loss of spherical appearance, dysplasia, osteonecrosis, subchondral fracture, cysts, or other lesions. Although the preoperative and intraoperative diagnoses by the surgeon are usually confirmed with pathological evaluation, in a significant portion of cases, additional findings by the pathologist can be discovered. In a recent study, discrepancy existed between the surgeon and histologic diagnosis in 18.4 percent of THAs and 9.4 percent of TKAs (6).
The pathologist should process the following samples:
Synovial tissue to assess the degree and type of inflammation.
The articular cartilage-bone interface to assess the subchondral bone and tidemarks.
A piece of the bone marrow to assess hematopoiesis.
Any abnormal gross areas such as cysts.
Revision of Total Hip Arthroplasty
Revision total hip surgery may be necessary for failure of total hip components. It can involve the replacement of one or all components. Each year, about 20,000 of these procedures are performed in the United States (2).
Indications
Failure may be secondary to aseptic loosening, osteolysis, instability, malalignment, component wear, periprosthetic fracture or infection, or normal “wear and tear” of the old implant (see Table 17.2, Chapter 17).
Procedure
The hip may be accessed through multiple approaches. Good exposure is required to adequately evaluate the joint and bony components. A trochanteric or extended trochanteric osteotomy is often utilized to remove the femoral component. This procedure separates the trochanter and lateral proximal femoral bone mass from a well-fixed prosthesis or intramedullary cement. Tools such as osteotomes (chisels), saws, burrs, curettes, and ultrasonic devices are used to explant the components. Attention is then turned to acetabular component removal.
Once the components are removed, preparation of the bone for implantation of revision components ensues. Modular trial components are used to assess sizing and stability of the final components to be used. Problems may arise during revision surgery, such as fractures of diaphyseal bone in the femur. For such cases, long-stemmed implants are available, which allow passage of the component distal to the fracture or cortical defect. In cases when an osteotomy is performed, the proximal fragment must be reduced to the distal fragment. They are held reduced with bone clamps or cables while preparation of the femur occurs. For cases in which significant acetabular bone was lost, the acetabulum can be augmented with mesh wire and screws along with allograft, or special cages preventing medial migration of the acetabular component. Once sizing with the trial implants is complete, these are removed and new implants are placed.
Pathology Specimens and Processing
Bone cuts, primary implants, and other soft tissue are sent to pathology, where they can be evaluated for causes of failure of the components. In septic cases, cultures are obtained from all components with ascertainment of the infecting organism and antibiotic susceptibilities.
Several samples of the soft tissue should be processed to ensure adequate and accurate counts of polymorphonuclear leukocytes in the assessment of possible periprosthetic joint infections. Any darkened tissue should be adequately sampled and processed to ensure a thorough assessment of wear debris. Whereas cement and polyethylene debris can lighten the tissue, metal debris can lead to a grayish or blackened appearance. The pathologist should use polarized microscopy to ensure adequate identification and
quantification of wear debris (see Table 17.5, Chapter 17). The type of inflammation (polymorphonuclear leukocytes vs. mononuclear cells vs. histiocytes) should be described to ensure adequate classification of the immune response to wear debris where present.
quantification of wear debris (see Table 17.5, Chapter 17). The type of inflammation (polymorphonuclear leukocytes vs. mononuclear cells vs. histiocytes) should be described to ensure adequate classification of the immune response to wear debris where present.
Partial Hip Replacement
Partial hip replacement, or hemiarthroplasty, in contrast to total hip replacement, is the replacement of the femoral head and neck only. This procedure is most commonly performed in the elderly, with low functional demand, in the setting of a femoral neck fracture in which fracture pattern or poor bone quality prohibits internal fixation. Just over 100,000 such cases are performed every year in the United States (2).
Indications
Typically, hemiarthroplasty is performed after a femoral neck fracture in the elderly, usually physiologically older than 70 years. The femoral neck fracture should be displaced and not amenable to internal fixation. Patients with poor bone quality are candidates for hemiarthroplasty, and the patient should not have significant acetabular disease. Hemiarthroplasty does lead to increased wear of acetabular bone, and patients are at increased risk of dislocation and infection.
Procedure
There are multiple approaches to the proximal femur, ranging from anterolateral to lateral to posterior. Different approaches differ upon where the joint capsule is being entered, which can lead to different complications and risks for each approach. They all involve incision of the capsule and exposure of the hip joint. Once the capsule is visualized and there is adequate surrounding exposure, the hip is dislocated, allowing visualization of the acetabulum, femoral head, neck, and proximal femur. A femoral neck cut is made, and the femoral head is removed. The size of the implant head is determined by templates. A reamer is used to enter the canal proximally, and excess cancellous bone is removed from the canal. A broach is then used in the proximal canal until sufficient resistance is encountered and appropriate fit of the broach is visualized. The size of the broach will determine the size of the implant. The appropriate trial (nonimplanted, test components) head and neck components are then inserted, and a test reduction is performed. Stability is tested in full flexion, extension, internal rotation, and external rotation, along with movement of the implant with traction. Once deemed stable, the final implants are inserted. Hemiarthroplasty can be performed using either a cemented or uncemented technique for fixation of the femoral component.
Pathologic Specimen and Processing
The specimens sent to pathology include the femoral head and neck, synovium, cartilage, and reamings. As discussed in THA, the head can be divided in observation of any abnormal anatomy, cysts, or other lesions. Typically, these specimens are sent when there is suspicion for a pathologic fracture.
The same processing strategy for THA is used for partial hip replacement surgery.
Total and Partial Shoulder Arthroplasty
Almost 40,000 total shoulder arthroplasties and 20,000 partial shoulder arthroplasties are performed yearly in the United States (2).
Indications for Shoulder Hemiarthroplasty
Shoulder hemiarthroplasty refers to replacement of the humeral neck and head with a prosthetic device. It is performed for the treatment of severe humeral head fractures. It can also be indicated for the treatment of arthritis, which is limited to the humeral head, where the glenoid articular surface usually has minimal arthritic changes.
Indications for Total Shoulder Arthroplasty
Total shoulder arthroplasty (Fig. 19.3) involves the reconstruction of the shoulder joint, usually to alleviate the symptoms of pain and limitations of activities of daily living in patients with arthritis. It may also be performed in patients with prior hemiarthroplasty in which the glenoid cartilage has worn down to bone, necessitating revision to total shoulder arthroplasty. Patients undergoing these procedures should have an intact rotator cuff and adequate glenoid bone stock.
Pain, especially at night, with limitations of activities of daily living, glenoid chondral wear down to bone, and posterior humeral subluxation are all indications for surgery. Usually, nonoperative measures such as oral analgesics, injections, and physical therapy have failed. If the patient is younger than 50 years of age and with greater physical function (e.g., weight lifting, strenuous activity) other modalities such as hemiarthroplasty should be considered.
Procedure for Total Shoulder Arthroplasty
When the patient is taken to the operative theatre, a dissection is carried down through the deltopectoral interval, and the extracapsular aspect of the proximal humerus is identified. The lesser tuberosity is divided from the proximal humerus. The anterior joint capsule is opened to expose the humeral neck, and all osteophytes are removed. Removal of the humeral head is achieved by cutting through the surgical neck of the humerus with a saw. A reamer and box osteotome are used to prepare the proximal humeral shaft for implantation of the prosthetic humerus. Trial implants, including the humeral shaft and head components, are assessed for size and stability, and final implantation is performed.
Attention is then turned to the glenoid preparation. This involves the concentric reaming of the glenoid surface. In cases of significant degenerative changes, where there may be instability posteriorly, the surgeon may use bone graft to augment the posterior glenoid in order to prevent posterior dislocation. Anchoring holes are created in the surface of the glenoid. A trial glenoid component is inserted, again to assess size and stability. The final implant is cemented into place in the glenoid.
Pathologic Specimen and Processing
Specimens sent to pathology include humeral and glenoid bone, cartilage and labrum, intramedullary reamings, and synovium. The surgeon typically expects the pathologic findings consistent with preoperative and intraoperative diagnoses, usually arthritis. In certain cases, osteonecrosis and, rarely, a neoplasm are also additional findings.
Samples of synovium, the fibrocartilaginous tissues, and bone should be processed to assess the type, degree, and extent of inflammation and degeneration.
Spine Procedures
Spinal Fusion
Spinal fusion involves the joining of multiple levels of the spine. It is commonly performed in the lumbar, lumbosacral, and cervical
spine. Indications for fusion may be spinal trauma, degenerative disc disease, disc herniation, scoliosis, and spondylolisthesis. About 430,000 such fusions at any level are performed yearly in the United States (2).
spine. Indications for fusion may be spinal trauma, degenerative disc disease, disc herniation, scoliosis, and spondylolisthesis. About 430,000 such fusions at any level are performed yearly in the United States (2).
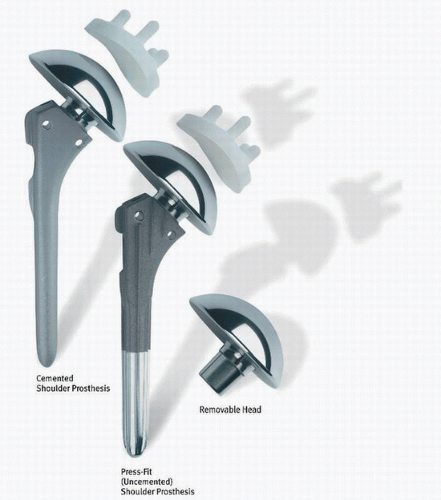 FIGURE 19.3. Modular total shoulder system. Cemented and uncemented shoulder prostheses, with modular humeral heads and polyethylene glenoid components. (Image courtesy of Zimmer.) |
Indications
The common indication for surgical intervention in many of the disorders is neurologic impairment. An example would be a patient with disc herniation in the lumbar spine presenting with radiculopathy along with objective weakness in the lower extremities. Sometimes, though, surgery may be indicated in patients with disabling pain secondary to disc herniation or degeneration that has failed a thorough course of nonoperative management such as physical therapy, pain management, and injection therapy consisting most frequently of a steroid in combination with a local anesthetic. In cases of severe back deformity, surgical fusion may be indicated for reconstruction of the spine to improve function. Trauma to the vertebrae may lead to fractures, causing instability of the spinal column, necessitating fusion.
Procedure
There are many approaches to spinal fusion, which are dependent on the region of the spine being fused and the reason for which fusion is being performed. Fusions may be performed either anteriorly or posteriorly, depending on the pathology being treated. In discussing anterior fusion, we will use cervical stenosis secondary to disc herniation as our example. In such cases, the diseased disc(s) is/are removed. The bony ends of the vertebrae are prepared and foraminotomies (removal of nerve-impinging bone or disc material from the intervertebral foramen) may be performed to ensure decompression of the nerves. Then, graft can be placed in the disc spaces for structural integrity. Finally, a plate can be placed across the vertebral bodies involved in the decompression, with screws placed into the vertebral body for fixation. If less than four vertebral levels have been decompressed, the surgeon may choose to forego instrumentation, in which case autograft is generally placed in the intervertebral spaces and becomes compressed due to the natural elasticity of the spine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




