(1)
G18, Royal Hallamshire Hospital, Sheffield, UK
Abstract
Glandular neoplasia of the cervix is a rare entity. Development of glandular neoplasia is associated with high risk human papillomavirus infection, most commonly type 18. The impact of cervical screening programmes in the detection and treatment of cervical glandular intra-epithelial neoplasia (CGIN) is unclear. Treatment of CGIN has become more conservative but concerns about skip lesions remain. High cure rates for CGIN by local excision are reported. Treatment of choice for early stage adenocarcinoma is surgery as radiotherapy may not be as effective compared with squamous cancers. Women with advanced adenocarcinoma of the cervix also have a worse outcome. Trials of new chemo-radiotherapy regimen are required.
Keywords
Glandular neoplasiaCervixAdenocarcinomaTreatmentSurgeryRadiotherapyIntroduction
The greatest challenge when managing women with cervical glandular neoplasia is the rarity of the condition thus preventing a clinician from gaining extensive experience in the subject. Most publications report small number of cases and so extrapolating findings must always be done with some degree of caution. Colposcopy is important in the diagnosis of cervical neoplasia but unlike squamous lesions glandular neoplasia are almost never recognized on colposcopic examination hence management is very dependent on referral cytology.
Cervical Glandular Intra-epithelial Neoplasia
Incidence
In the year 2011/12 the English cervical screening programme reported 1,354 samples as showing ?glandular neoplasia out of a total of 3,457,752 cytology samples taken that year. Thus only 0.6 % of all the reported abnormal cytology samples fall within this category. During the same year only 0.8 % of the women referred to colposcopy were referred because of ?glandular neoplasia and of those who subsequently underwent excisional treatment for cervical neoplasia only 2.1 % of excised specimens contained high grade- cervical glandular intra-epithelial neoplasia (HG-CGIN) [1].
We know little about the incidence of borderline changes in endo-cervical cells within the English screening programme. Although well recognized by cytologists and colposcopists, this category was not recorded as an entity separate from squamous borderline changes. This situation has been rectified with publication of NHSCSP No1 3rd ed. ABC document in April 2013 and in future years we will be able to gain more accurate data on the incidence of this cytological diagnosis [2].
Aetiology
It is generally accepted that glandular neoplasia develops at the squamo-columnar junction rather than developing in endo-cervical cells high in the endo-cervical canal. Ibrahim et al. looked at cellular markers expressed at the squamo-columnar junction and found that the same population of reserve cells gives rise to both glandular and squamous neoplasia [3]. Cervical glandular neoplasia is associated with high risk HPV infection. HPV 18 is more commonly associated with this lesion as described in Chap. 3. It is unclear why HPV 18 may preferentially favour infection of endo-cervical reserve cells leading to glandular neoplasia. The fact that high risk HPV infection of the reserve cell population is common to the development to both squamous and glandular explains why many women with glandular neoplasia have co-existent cervical intra-epithelial neoplasia (CIN). In addition some women who are eventually found to have glandular neoplasia may have been referred to colposcopy with a screening cytology reporting only changes in squamous cells.
The Role of Colposcopy in the Diagnosis of Cervical Glandular Intra-epithelial Neoplasia
Glandular neoplastic lesions are located within the columnar epithelium of the endo-cervix so hampering full visualization of the entire at risk area by colposcopy even with the use of endo-cervical speculae. Features of cervical glandular intra-epithelial neoplasia (CGIN) are only present after the application of acetic acid. The two most commonly described changes are the presence of dense aceto-white columnar villi, especially if these changes are not adjacent to the squamo-columnar junction, and the finding of villi fused together (Figs. 8.1 and 8.2). Mosacisim or punctation are not usually found but atypical blood vessels can be seen and may raise the possibility of a micro-invasive cancer. Unfortunately these features are not always present and hence considered unreliable implying that colposcopists should not rely on the few features that have been described to either confirm or exclude the presence of CGIN [4, 5]. Between 47 and 87 % of women with pure HG-CGIN may have a normal colposcopic examination [6, 7]. These finding are similar to those reported by Pisal et al. who also reported with only 1 out 13 cases of HG-CGIN was diagnosed colpscopically [8]. National U.K. colposcopy guidance states that directed biopsies should not be used in the management of these cases [9]. Unlike squamous intra-epithelial neoplasia it is also not possible to assess if a lesion is low or high grade based on colposcopic features described above.
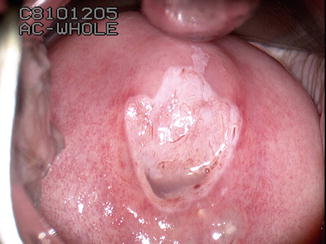
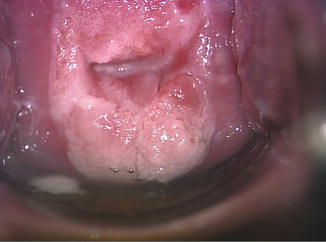

Fig. 8.1
Dense aceto-white changes affecting the columnar epithelium with fusion of the villi and abnormal blood vessel pattern

Fig. 8.2
Irregular lesion with the columnar epithelium with dense aceto-white changes
Although colposcopy cannot confirm or refute the presence of CGIN useful information can still be obtained as a result of the examination. The presence of any invasive cancer, as long as it is low in the endo-cervical canal, can be detected. Adenocarcinoma of the cervix shares the same features as squamous cancer; irregular surface either raised or ulcerated the presence of atypical blood vessels. The presence of any co-existing squamous lesions can be assessed and this may impact on how the abnormality is treated.
Management of Women with? Endo-cervical Glandular Neoplasia
Diagnosis
All women with an abnormal cytology sample showing ?glandular neoplasia should be referred to colposcopy. All reported series show high rates of significant disease including invasive cancer. Older studies also found cases of cancer of the endometrium, upper genital tract and other abdominal organs [5]. Modern classification system introduced over recent years has encouraged cyto-pathologists to try and differentiate endo-cervical glandular neoplasia from other glandular neoplasia.
Ullal et al. examined the performance of cytology and colposcopy in women with endo-cervical neoplasia [6]. While cytology had both a reasonable sensitivity (66 %) and positive predictive value (81 %) (PPV) colposcopy had only 10 % sensitivity but a high PPV (94 %). Even when colposcopy was reported as normal over 87 % had a significant lesion on the subsequent excised specimen. Talaat et al. reviewed 200 cases referred with ?glandular neoplasia over a 10 year period [7]. Pure HG-CGIN was a relatively rare finding with only 14 cases found out of 115 cases on intra-epithelial neoplasia, the most common finding was combined HG-CIN and HG-CGIN, 70 cases, and 30 cases of HG-CIN. 48 women were found to have invasive cancer, 28 cervical adenocarcinomas, 8 squamous carcinomas, 10 endometrial cancers and two ovarian cancers. The high incidence of significant disease within this category has led to the English cervical screening programme recommendation that women with this cytology abnormality should be seen in colposcopy within 2 weeks.
Endo-cervical Curettage
There are significant differences of opinion as to the role of endo-cervical curettage in the management of women with CGIN, while it is common practice in the U.S.A.; it is rarely performed in the U.K. The poor sensitivity associated with this technique linked to a variable but high rate of false negatives (59–78 %) has influenced the decisions of U.K. colposcopists not use this form of sampling [10–12]. In addition endo-cervical curettage is uncomfortable.
Treatment
The treatment of HG-CGIN must be by an excisional technique, which may be large loop excision of the transformation zone (LLETZ, LEEP), laser conisation or cold knife conisation. There is a wide range of opinion as to which method is best. It is best to try and remove a cylinder of tissue rather than a cone to minimize the risk of cross cutting the endo-cervical glands leading to an increased rate of incomplete excision. Some colposcopists favour using cold knife conisation as it does not damage the delicate endo-cervical tissue at the excision margin so providing better quality tissue for pathological assessment. However, it can be difficult to minimize the size of the tissue excised using this technique leading to concern about removing too much tissue from the cervix. In addition this technique requires general or regional anaesthesia unlike laser conisation or LLETZ, which can be performed under local anaesthesia in a clinic setting. The depth of the excised tissue should be at least 1 cm above the squamo-columnar junction (SCJ) if it is visible, if the SCJ is not visible the depth of the excised tissue should be 2–2.5 cm. The depth of the excised tissue is significantly greater than for CIN, 0.8 cm if the SCJ is visible, or 1.5 cm is the SCJ is not visible. Van Hanegem et al. 2012 reviewed the management of 112 women treated for adenocarcinoma in-situ (HG-CGIN) with either cold knife cone biopsy or LLETZ [13]. The rate of negative margins was the same in both groups (79 % vs. 73 %). All women with positive margins underwent a further excision and the rate of residual disease was the similar in both groups (33 %). Some women with negative margins underwent re-excision but with no evidence residual disease. The depth of the excised specimen was much greater in the knife cone biopsy group but this did not reduce the incidence of positive margins. The use of LLETZ may be of greater benefit for young women, although some will need a second procedure the majority will be cured by the first excision.
Some experts remain concerned about the possibility of skip lesions; hence consider that negative margins within the excised specimen cannot completely exclude residual disease within the endo-cervical canal. Therefore there is still a role for hysterectomy as the primary management of women who present with glandular neoplasia and have completed their family. Some however recommend considering hysterectomy as completion of treatment once they have completed their family if they had earlier undergone conservative local excision. If follow-up cytology was normal during the interval between local excision and completion of their family the benefits of hysterectomy would only be to reduce the development of new disease rather than excision of any residual occult HG-CGIN.
Management of Women with Borderline Changes in Endo-cervical Cells
All women with an abnormal cytology sample showing borderline changes in endo-cervical cells should be referred to colposcopy. Unlike borderline nuclear changes in squamous cells there is a high incidence of abnormality, both squamous and glandular, associated with borderline changes in endo-cervical cells. In 2011 the English cervical screening programme introduced high risk HPV triage for all cytology samples reported as borderline, either squamous or glandular, and mild dyskaryosis. It is as yet unclear what effect this will have on the referral pattern as the English programme only started to record borderline changes in endo-cervical cells as a separate category from squamous borderline changes in April 2013 following publication of guidance from NHSCSP publication No1 (ABC) [2]. Studies have reported the presence of significant disease with in the category but rarely HG-CGIN. Patel et al. reported that 32 % of women had at least squamous high-grade intra-epithelial neoplasia (HG-CIN) and 7 % had invasive cancer but only 4 % had HG-CGIN [8]. Other studies have reported the presence of high grade squamous lesions (10–33 %), invasive disease (1.8–22 %) and CGIN (10 %) [5, 14–17]. These studies are often small case series from individual colposcopy clinics or cytology laboratories using a variety of different cytological classification systems and hence must be viewed within this context.
Colposcopic examination will aid the diagnosis of invasive disease and CIN however it cannot confirm or exclude CGIN. Given the very low incidence of CGIN in this group a conservative approach to management is a reasonable option. Repeat colposcopic examination with cytology sampling should be undertaken 6 months later. If the repeat cytology is reported as showing borderline changes in endo-cervical cells excision of the transformation zone should be considered to confirm or exclude the presence of HG-CGIN.
The Role of Repeat Excision
Unlike squamous CIN where there is a lot of information about factors that influence repeat excision the data for CGIN is less abundant. Older data suggested that there was a high incidence of residual disease because of skip lesions but more recent studies suggest that the risk is much lower. Young et al. reported that the risk of residual disease was 55 % if initial excision margins were positive but only 13 % if they were negative [18]. In a large meta-analysis of 1,278 women treated for CGIN the incidence of residual disease was only 2.6 % in women with negative margins [19]. Kurina and al-Nafussi examined 121 cases of CGIN and found that if there was at least 3 mm of normal endo-cervical epithelium between the CGIN and the excision margin then there was no evidence of residual disease [20]. Li and Zhao in a recent review of 136 cases of AIS (HG-CGIN) found that at the time of hysterectomy the incidence of residual disease was 0 % if the prior conisation specimen had negative margins. There was only one case of recurrent disease in this group. In the group of women with positive cone margins 48.6 % had residual disease at hysterectomy [21]. The above data has confirmed that conservative management of completely excised CGIN is acceptable and now become the standard of care.
Management of Squamous Mucin Intra-epithelial Lesion (SMILE)
Although this is a variant of squamous intra-epithelial neoplasia it can be found co-existing with CGIN. There are no colposcopic features described for SMILE and it is best managed according the co-existing CIN or CGIN.
Follow Up of Women Treated for CGIN
All British cervical screening programmes recommend cervical cytology follow-up after treatment for CGIN. Concerns over the sampling and shedding of the endo-cervical canal has resulted in this group of women being excluded from using high risk HPV testing within the test of cure setting. Kitchener et al. reported a case of adeno-carcinoma that developed in a woman despite having negative cytology and negative for HR-HPV [22]. Intensive cytology follow-up, 6 and 12 months and a further 9 annual cytology samples is recommend. The presence of endo-cervical cells is a mandatory of the sample if it is to be reported as negative. In situations where the endo-cervical canal is stenosed due to conisation an additional endo-cervical brush sample may be required if the ecto-cervical brush cannot access the higher portion of the canal. A recent development in the English Cervical Screening programme has seen this recommendation change. Women will now have two samples taken, six and then 18 months after treatment. If the cytology is negative reflex HR-HPV testing will take place. If both samples are negative for both cytology and HR-HPV then the woman will be returned to normal re-call.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


