Chapter 5 Coenzymes
Adenosine triphosphate has two energy-rich bonds
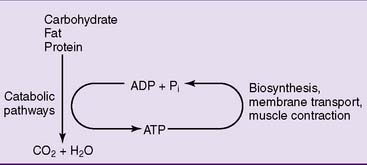
Metabolic energy is generated by the oxidation of carbohydrate, fat, protein, and alcohol. This energy must be harnessed to drive endergonic chemical reactions, membrane transport, and muscle contraction. Nature has solved this task with a simple trick: Exergonic reactions are used for the synthesis of the energy-rich compound adenosine triphosphate (ATP), and the chemical bond energy of ATP drives the endergonic processes. In this sense, ATP serves as the energetic currency of the cell (Fig. 5.1).
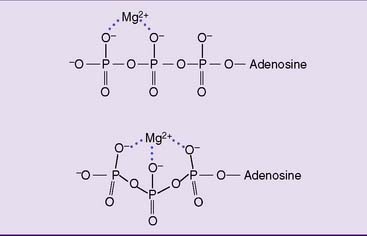
ATP is a ribonucleotide, one of the precursors for ribonucleic acid (RNA) synthesis. It does not contain a vitamin, and the whole molecule can be synthesized from simple precursors (see Chapter 28). Its most important part is a string of three phosphate residues, bound to carbon 5 of ribose and complexed with a magnesium ion (Figs. 5.2 and 5.3).
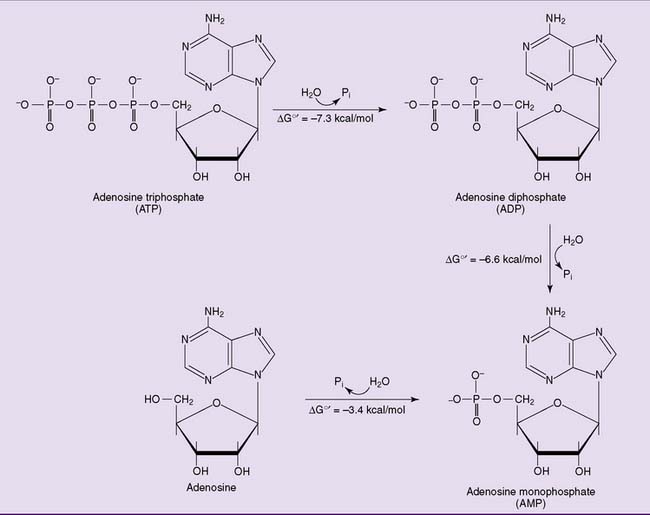
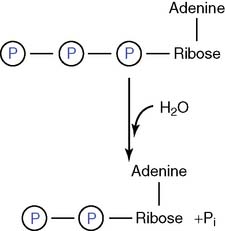
Figure 5.2 Sequential hydrolysis of ATP. ΔG0′, Standard free energy change; Pi, inorganic phosphate.
The first phosphate is linked to ribose by a phosphate ester bond, but the two bonds between the phosphates are energy-rich phosphoanhydride bonds. The free energy changes shown in Figure 5.2 apply to standard conditions. The actual free energy change for the hydrolysis of ATP to ADP + inorganic phosphate (Pi) depends on pH, ionic strength, and the concentrations of ATP, ADP, phosphate, and magnesium. It is close to −11 or −12 kcal/mol under “real-cell” conditions. ATP can be hydrolyzed to ADP and phosphate:
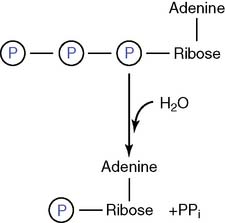
or to adenosine monophosphate (AMP) and inorganic pyrophosphate (PPi):
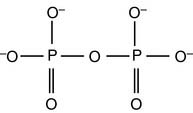
The PPi formed in the second reaction still contains an energy-rich phosphoanhydride bond:
ATP is the phosphate donor in phosphorylation reactions
Table 5.1 lists the most important uses of ATP. Only phosphorylation reactions and the coupling to endergonic reactions are considered here.
| Process | Function |
|---|---|
| RNA synthesis | Precursor |
| Phosphorylation | Phosphate donor |
| Coupling to endergonic reactions | Energy source |
| Active membrane transport | Energy source |
| Muscle contraction | Energy source |
| Ciliary motion | Energy source |
RNA, Ribonucleic acid.
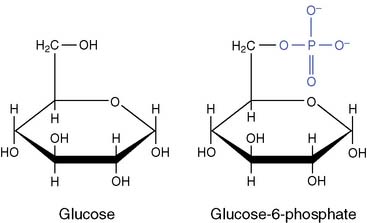
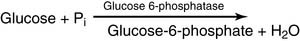
One possibility is to synthesize glucose-6-phosphate by reacting free glucose with Pi:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree