Chapter 12 Clostridium Difficile Infection
Definition and Epidemiology
Definitions
Clostridium difficile is an anaerobic, spore-forming gram-positive bacillus. C. difficile causes a diarrheal infection, which accounts for virtually all cases of pseudomembranous colitis, and 20% of antibiotic-associated diarrhea. The fastidious spores may persist for years in the environment. C. difficile produces two toxins, Toxin A and Toxin B, which are cytotoxic and cause colonocyte necrosis. Nontoxin producing strains are not pathogenic.
Incidence
C. difficile diarrhea (CDD) is the most common nosocomial enteric infection. An estimated 300,000 to 3 million new cases occur per year, prolonging inpatient stays by an average of 2 weeks, and increasing hospitalization costs by $6000 to $10,000 per case.
Despite the high inpatient incidence rates, CDD remains an uncommon outpatient problem. The incidence rate is approximately 7.7 cases per 100,000 person-years, or about 20,000 new cases per year. However, many experts believe that CDD is underdiagnosed in the outpatient setting.
Risks
Inpatient Hospitalization
Colonization by C. difficile is directly related to the duration of hospital stay. Patients hospitalized for less than 1 week have a colonization rate of 1%, whereas those hospitalized for 4 or more weeks have a colonization rate of greater than 50%.
Sources of spread of C. difficile include infected or colonized roommates, carriage on personal items of physicians, and fastidious spores found in the patient’s room. Therefore, enteric isolation precautions, room disinfection, and handwashing remain essential for preventing CDD outbreaks (Box 12-1).
Box 12-1 General Principles of Clostridium difficile Therapy
General Principles
C. difficile Colitis with or without Pseudomembranes
Antibiotic Use
Antibiotic use is another major risk factor for the acquisition of CDD. The overall risk of CDD is 6.7 cases per 100,000 risk-exposures (defined as 42 days). Antibiotics commonly associated with the development of CDD are clindamycin, ampicillin, amoxicillin, and cephalosporins. Tetracycline, metronidazole, intravenous vancomycin, chloramphenicol, and aminoglycosides have a lower risk for the development of CDD. Other antibiotics carry an intermediate risk for causing CDD.
Rarely, CDD may be associated with the use of chemotherapeutic agents, particularly methotrexate and paclitaxel.
Carrier State and Colonization
Three percent of otherwise healthy adults are carriers of C. difficile. Two thirds of patients colonized by C. difficile remain asymptomatic. These persons may be at a lower risk for the development of symptomatic CDD (described in more detail in the following text).
Other Risk Factors
Other risk factors for CDD reflect underlying host factors. Elderly or malnourished patients and patients with severe concurrent illness are at higher risk for the development of CDD.
In addition, some studies suggest that the use of proton pump inhibitors may increase the risk of CDD in certain patients.
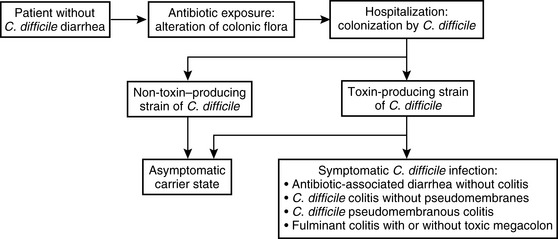
Pathogenesis
Step 1—Exposure to Antibiotics
The first step in the development of CDD is exposure to antibiotics. Antibiotics alter colonic flora, allowing for colonization by C. difficile. Although different antibiotics have varying risks for the development of CDD, broad-spectrum antibiotics are usually associated with a higher risk for CDD.
CDD usually develops between days 4 and 9 of an antibiotic course, but has been seen up to 8 weeks following the cessation of therapy.
Step 2—Colonization by C. difficile
Fecal–oral acquisition of C. difficile, usually during inpatient hospitalization, leads to colonization, with rates as high as 50% after 4 weeks.
Step 3—Development of C. difficile Diarrheal Illness:
After colonization, approximately two-thirds of patients become asymptomatic carriers and the remaining one third develop symptomatic CDD. Although the precise reason why some patients develop CDD is unknown, the interactions between C. difficile and intrinsic host factors have been implicated.
The development of an antibody response has been shown to be protective against the development of CDD and recurrent CDD. Persons with titers of anti-Toxin A IgG titers less than three ELISA units have an odds ratio of 48 for the development of CDD. Similarly, a systemic IgM response on day 3 and IgG response on day 12 were associated with a decreased risk for recurrent CDD.