Fig. 4.1
Different sizes of blisters in a hand of Iranian soldier 3 days after direct SM skin exposure during the Iraq Iran war (An unpublished slide of the corresponding author’s collections that was taken with permission of the patient under his care)
4.5.2 Gastrointestinal Symptoms
The gastrointestinal symptoms of acute phase in SM intoxication during the first week after exposure in Iranian veterans were nausea (47 %), vomiting (42 %), anorexia (40 %), abdominal pain (35 %), diarrhea (15 %), melena (7 %), and hematemesis (5 %) (Noorbakhsh and Balali-mood 1994). Endoscopy and physical examination show that acute esophagitis and gastritis may be diagnosed in patients who had ingested contaminated foods. Erosion and blisters as common irritations in the pharynx, esophagus, and stomach may also be observed in these patients. But, common GI chronic symptoms 2 months to 6 years after exposure were nausea (45 %), anorexia (42 %), abdominal pain (38 %), hematochezia (12 %), hematemesis (5 %), gastritis (12 %), duodenitis 8 %), and duodenal ulcer (1 %) (Noorbakhsh and Balali-mood 1994).
4.5.3 Respiratory Symptoms
Coughing, expectoration, dyspnea, hemoptysis, wheezing, crackles, stridor and hypoxemia are major SM-induced respiratory clinical manifestations (Balali-Mood et al. 2005b, 2011). The most important toxicity on respiratory system in Iranian veterans intoxicated with SM included obstruction of upper airways, chronic bronchitis, bronchiectasis, asthma, emphysema, stenosis of large airways and pulmonary fibrosis (Ghasemi-Boroumand et al. 2007). Other pathological effects on the respiratory system were chronic laryngitis, thickening of the bronchial walls, hoarseness of voice, tracheobronchitis, recurrent respiratory infections, acute respiratory failure, laryngeal carcinoma, and lung cancer (Razavi et al. 2012). Also, pulmonary edema was the main cause of death among the soldiers who died a few days after exposure to SM (Freitag et al. 1991).
4.5.4 Acute Effects in the Eyes
Eyelid edema, limbal ischemia, limbal pigment loss, blepharospasm, chemosis, conjunctivitis and retina ulcer were the most frequent ocular manifestations due to SM poisoning. Blurred vision, visual impairment, scarring and neovascularization of the anterior chamber has also been reported among Iranian veterans. Other ocular complications include corneal opacity, keratitis, uveitis, corneal melting, conjunctivalization, perforation, and blindness in rare cases (Ghasemi-Boroumand et al. 2007; Razavi et al. 2012). A picture taken from a left eye of a patient taken 5 days after SM exposure during the Iraq Iran war that revealed blepharospasm, chemosis, keratitis with severe eye vision deficit is shown in Fig. 4.2.
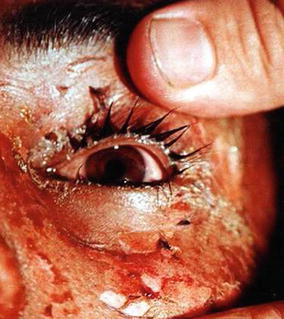

Fig. 4.2
A picture taken from a left eye of a patient taken 5 days after SM exposure during the Iraq Iran war, showing blepharospasm, chemosis, keratitis with severe eye vision deficit (An unpublished slide of the corresponding author’s collections that was taken with permission of the patient under his care)
4.6 Chronic Intoxication of Mustard Gas
Chronic toxicity of SM intoxication often occur when manual workers who are chemically exposed to mustard agents in factories that produce these agent (Balali-Mood et al. 2008). Although the chronic and delayed toxicity of mustard gas typically occur in the affected organs of acute toxicity, but some chronic and long-term complications may also occur in organs which are less affected in acute intoxications (Panahi et al. 2013; Amirzargar et al. 2009).
Because the skin is the largest organ exposed to these chemical agent, it is supposed to be more susceptible to these compounds than any other organs. The most common skin complications of SM toxicity are abnormal skin pigmentation and dry skin (Balali-Mood et al. 2005a, b). On the other hand, some findings showed that even after decades of exposure to SM, severe long-term respiratory disease such as obstructive pulmonary disease (COPD), lung fibrosis and can occur. Other chronic respiratory disorder due to SM poisoning include bronchitis, bronchiectasis, asthma and interstitial fibrosis (Balali-Mood et al. 2005b).
Liver and other internal organs, which are less likely to be directly affected by mustard gas are mostly involved in chronic and delayed toxicity (Kazemzadeh et al. 2014). It seems that the liver is also affected organ in chronic SM poisoning (Vijayaraghavan et al. 2005). Liver function evaluation of Iranian veterans with mustard gas poisoning showed that chronic hepatitis and steatosis were the most common pathologies (Kazemzadeh et al. 2014).
Vascular tortuosity, chronic conjunctivitis, corneal vascularization, corneal thinning, corneal opacity, corneal epithelial defect and cornea melting are considered as the most common long-term complications of the eyes due to exposure to mustard gas (Javadi et al. 2005; Balali-Mood et al. 2005b).
Although thrombocytopenia, anemia and leukocytosis are the most common hematological complications soon after exposure to SM, long-term studies on Iranian veterans 16–20 years after exposure revealed no significant hematological abnormalities. Total counts for WBC and RBC, percentages of monocytes and CD3+ T-lymphocytes, the level of IgM, absolute levels and percentages of α2 and β globulins and C3 levels were slightly high in the intoxicated patients compared to the count in healthy controls (Mahmoudi et al. 2005). Depression of the cell-mediated immunity is reported as an important long-term effect of SM poisoning. Also, the reduction of helper T cells and increased level of T suppressors may result from chronic immunotoxicity of SM intoxication (Zandieh et al. 1990). A report showed that natural killer cells are impaired in patients with occupational exposure to SM (Yokoyama 1993). Studies on Iranian veterans also revealed that the percentage of natural killer cells almost 10 years after exposure to mustard gas is significantly lower than that of the healthy individuals (Ghotbi and Hassan 2002). Also it is shown that immunotoxicity and hematological complications are mainly due to systemic toxicity (Balali-Mood et al. 2005a).
4.7 Delayed Toxic Effects of Mustard Gas
According to the studies conducted on chemically injured war victims, the most common late clinical complications due to exposure to SM were observed in the respiratory tract, neuropsychiatrics systems, the skin, and eyes with the frequency of 78 %, 45 %, 41 %, and 36 %, respectively (Balali-Mood 1992; Balali-Mood and Navaeian 1986). Delayed toxicity of mustard agents typically refers to genotoxicity, carcinogenicity and adverse reproductive effects. Due to their alkylating potential, MCs are mutagenic and genotoxic materials (Fox and Scott 1980).
4.7.1 Long-Term Dermal Complications
Dry skin, burning sensation, itching, atrophy and hypo and hyper pigmentation in the skin are the most important late dermatologic complications of exposure to mustard gas (Hefazi et al. 2006). Some important skin injuries in Iranian veterans exposed to mustard gas include hyperpigmentation, erythematous popular rash, dry skin, multiple cherry angiomas, atrophic scar, hypopigmentation, hair loss and hypertrophy. As shown in Figs. 4.3 and 4.4, other microscopic skin changes include epidermal atrophy, hyperkeratosis, basal membrane hyperpigmentation, non-specific dermal fibrosis, melanocytes and melanosomes within epidermis and increased collagen fibers and mononuclear inflammatory cells within dermis (Balali-Mood et al. 2005b).
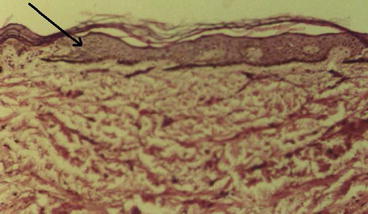
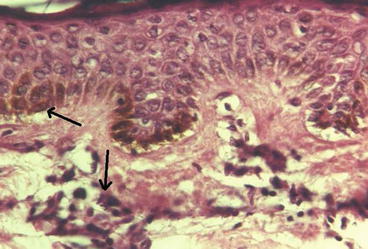

Fig. 4.3
Orthokeratotic hyperkeratosis, and atrophy of appendices (Light Microscopy: 10 × 10, Courtesy of Prof. Tabatabei, MUMS, Iran)

Fig. 4.4
Basal membrane hyperpigmentation with mononuclear infiltration (Light Microscopy: 20 × 10, Courtesy of Prof. Tabatabei, MUMS, Iran)
4.7.2 Delayed Respiratory Effects
Coughing, expectoration, dyspnea, and hemoptysis are the most important and common symptoms of delayed respiratory complications. Other important long-term clinical findings in patients intoxicated with SM are wheezing, crackles and stridor (Balali-Mood et al. 2005b). Moreover, lung cancer and pneumonia is usually common cause of death from exposure to mustard gas (Manning et al. 1981). The results of a study conducted in Britain during 1961–1940 on mustard gas producers showed that the incidence of death from laryngeal cancer in this population has increased (Easton et al. 1988). Infection of the airways that leads to bronchopneumonia can also lead to death. A report showed that respiratory illness may worsen over time, whereas ocular lesions and skin damages may be declined (Hefazi et al. 2005). It was also reported that the severity of bronchiectasis exacerbates in long-term follow-up (Balali-Mood and Hefazi 2006).
4.7.3 Delayed Eyes Effects
Itching, burning sensation, red eyes, blurred vision, vision loss, photophobia and tearing are the most common recorded delayed eyes symptoms (Naderi et al. 2014). Corneal thinning, neovascularization and epithelial defects are other major ocular complications 16–20 years after initial exposure to SM (Etezad-Razavi et al. 2006). Study on 500 male patients intoxicated with mustard gas 15 years after initial exposure in the Iraqi–Iran conflict (1980–1988) showed that the patients may exhibit different grade of ocular complications (Ghassemi-Broumand et al. 2004).
4.7.4 Reproductive System
Animal data showed that exposure to SM may cause damage to the reproductive system in mice due to the inhibition of spermatogenesis (Graef et al. 1948). Although, due to high regenerative capacity of germ cells, it is theoretically suggested that the performance of reproductive system or the fertility may not be affected (Balali-Mood et al. 2008). However, studies on both humans and animals showed that as long-term complications of mustard gas can lead to impaired spermatogenesis even decades after exposure (Safarinejad 2001). A study on the SM veterans showed that semen indices are significantly lower in patients exposed to SM during Iran-Iraq conflict compared to normal healthy individuals. Therefore, SM can be considered as a gonadotoxic warfare agent (Amirzargar et al. 2009).
4.7.5 Cardiac Associated Complications
Clinical findings suggest that the incidence of coronary artery disease (CAD) and angiographic changes may increase due to exposure to SM. The myocardium of patients with mustard gas poisoning is pale and seems do not work properly, but no heart abnormalities are reported in these patients. Other cardiac associated anomalies such as left ventricular (LV) diastolic abnormality, myocardial perfusion and dilated cardiomyopathy may also observed in patients exposed to high dose of mustard gas (Rohani et al. 2010).
4.7.6 Genotoxicity
Also, because most of the complications occur several years after exposure to mustard agents, it is now suggested that genotoxicity and epigenetic abnormalities may be involved in delayed toxic effects of mustard gas. For example, sister chromatid exchanges in the peripheral lymphocytes are reported in patients exposed to SM (Aasted et al. 1987). Varieties of enzymes are involved in epigenetic changes including DNA methyltransferases (DNMTs), histone acetyl transferases (HATs) and histone deacetylases (HDACs), which cause chromatin changes leading to altered gene expression (Miremadi et al. 2007; Kunak et al. 2012). Exposure to mustard gas is also shown to be associated with almost 400 transcriptional changes, which may lead to DNA damage, cell cycle arrest, cell death and inflammation (Jowsey and Blain 2014).
In a study of Behravan et al. (2013), shorter telomere length and increased level of marker of oxidative stress in SM exposed veterans were found. They concluded that the findings confirm delayed genotoxicity of SM in patients over 25 years after single exposure to SM.
4.7.7 Carcinogenicity
Based on numerous evidences, it is obvious that mustard gas is a potent carcinogen in humans (Watson et al. 1989). It may causes cancer of the lung and cancer of the larynx (Easton et al. 1988). It is suggested that the carcinogenicity of mustard gas may be mediated by a genotoxic mechanism of action that involves DNA alkylation, loss of DNA repairing potency, mutations, and induction of chromosomal alterations ((IARC) 1999). Studies on workers of the Ohkunojima poison gas factory show that exposure to mustard gas is associated with high incidence of mutation, chromosome abnormality, sister chromatid and cytogenetic changes and missing Y chromosomes (Shakil et al. 1993). Therefore, it is suggested that exposure to mustard gas in long-term may lead to incidence of malignant tumors such as respiratory tract cancer and leukemia (Yanagida et al. 1988).
Because the mechanism of action in acute and delayed toxicity may differ, clinical manifestations may also differ in SM induced toxicity. Based on the documents that have been mostly obtained by the studies on the Iranian chemical warfare victims, some major clinical features of SM intoxication are summarized in Table 4.1.
Table 4.1
Major clinical features of SM intoxication in acute, chronic and delayed toxicity
Organs | Major clinical features | ||
|---|---|---|---|
Acute | Chronic | Delayed | |
Skin | Blister formation, vesication | Dryness, pigmentation | hyper pigmentation, popular rash, atrophic scar erythematous |
Eyes | Tearing of the eyes, corneal neovascularization | Chronic conjunctivitis, corneal thinning, corneal opacity | Blurred vision, visual impairment, keratitis, uveitis |
Respiratory system | Coughing, wheezing, bronchiectatic lesions | Asthma, bronchiectasis, obstructive pulmonary disease | Coughing, expectoration, dyspnea, and hemoptysis, pneumonia |
Gastrointestinal system | Mucosal necrosis, bloody diarrhea, vomiting, nausea | Nausea, abdominal pain, anorexia | – |
Immune system | – | Depression of immunity, reduction of natural killer cells | Reduction of natural killer cells |
Hematological system | Leukocytosis, hemorrhage | Thrombocytopenia, anemia | Anemia |
Neuro-psychiatric disorders | Convulsions | – | – |
4.8 Treatments of SM Poisoning
It was shown that SM may cause irritations in a few minutes after exposure, but no specific treatment is still available (Sidell et al. 1997). Physical blister removal, removing the contents of the blisters in the skin, and administering topical antibiotics have been widely used for the treatment of cutaneous SM injuries. Although, therapeutic potentials of several anti-inflammatory drugs, antioxidants, protease inhibitors and antiapoptotic compounds for SM induced injuries have been investigated; no significant progress for producing an efficient antidote has been obtained so far (Gu 2014; Poursaleh et al. 2012).
The healing rate of wounds induced by mustard gas is considerably slow, and typical treatment is almost symptomatic and supportive (Rice 2003). Effectiveness of anti-inflammatory drugs on SM induced skin injuries has been confirmed in an animal model. Data showed that anti-inflammatory drugs such as Adexone can relieve pain and reduce inflammation and the level of prostaglandin E. Researches also showed that non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac (Voltaren) do not have such therapeutic effects on SM induced skin damages, but combination of these drugs may potentially increases the chance of healing (Dachir et al. 2004).
Pharmacologically, no specific antidote is known for SM poisoning, but as previously described, the treatment to SM toxicity is typically symptomatic and supportive (Graham et al. 2005). Nonetheless, medical management of SM poisoning includes primary and secondary prevention (Poursaleh et al. 2012). Primary prevention is typically the promotion of healthcare through first-aid protection, control of pain and potential antidotal treatment with 100–500 mg sodium thiosulphate per kilogram body weight (Balali-Mood and Hefazi 2005b). Secondary prevention includes treatment with antibiotics and conventional therapies. Depending on the organ involved in acute SM poisoning, several medical management may be applied. For example, bronchodilators, corticosteroids, immunosuppressive agents, antibiotics, and oxygen therapy is often used in SM induced lung injuries (Vojvodic et al. 1985). Also several agents such as cysteine, sodium citrate, promethazine, heparin and vitamin E are shown to have protective effects against SM induced lung injuries. But, removing the victims from the contaminated areas is always the first step in treatment of veterans exposed to mustard gas.
To prevent further pollution, decontamination of clothes, skin, hair, and eyes with proper solution is required prior to therapy of SM intoxication. Decontamination efficiency of N, N′-dichloro-bis (2, 4, 6-trichlorophenyl) urea (CC-2) and Fuller’s earth (BPC standard) combination is shown in dermal intoxication in animal model (Kumar et al. 2013). For skin decontamination, great amounts of water and 0.5 % hypochlorite solution is recommended. For treating eyes, it should be noted that prior to decontamination and washing, the eyes should not be bandaged.
Although the main medical management in SM poisoning is almost standard first aid treatment, A study showed that thiol containing compounds such as N-acetyl cystine (NAC) and dimercapto succinic acid (DMSA) can have protective effects in SM induced injuries (Balali-mood and Anari 1989). Animal studies also suggest that some antioxidant and anti-inflammatory agents such as sodium thiosulfate, N-acetyl-L-cysteine, nicotinamide, nicotinic acid, promethazine, dexamethasone, prednisone, and vitamin E may have supportive effects and decrease tissue damage (Vojvodic et al. 1985; Dabney et al. 1991). Using poly (ADP-ribose) polymerase inhibitors, calmodulin antagonists and Ca2+ chelators are also suggested for primary care in lung injuries (Merat et al. 2003).
(a)
The skin
Nowadays extensive research program has started to provide new medical managements and therapeutic strategies for cutaneous pathology and blister formation caused by mustard gas poisoning. Because of the most important complication followed by SM exposure is cutaneous and respiratory injury, development of topical skin protectants and medical management for wound healing is of great interest and importance. Several months is often needed to cure skin injuries due to mustard gas exposure. Epithelium, dermal collagen and other connective tissues are affected and do not have normal functions in SM poisoning (Graham et al. 2009). Similar to other skin injuries such as severe burning and diabetic foot ulcers, debridement particularly laser debridement of lesion and blister is usually a successful strategy for treating SM induced dermal injury prior to any pharmacological treatment. Another report showed that the lesion is significantly lower when debridement is performed with trypsinlinked gauze than surgical or laser debridement (Eldad et al. 1998). From pharmacological aspect, compounds that are used to treat skin lesions, should increase the oxygen supply to the tissue, and stimulate epithelium to grow. For example, amino-Plex® is considered as a nutritive cosmeceutical product that increase oxygen in cells, improve glucose transportation, stimulate collagen formation, and promote angiogenesis (Graham et al. 2009).
Treatment of skin lesions in Iranian veterans for the chemical burns, using sulfadiazine in hydroxyl propylmethyl cellulose and furacine showed some beneficial effects. It is recommended to avoid the use of wet-to-dry dressings, and the wound is better to keep in moist condition. Unless for primary dressings, which shall be remained for at least a week, secondary bandages is ought to be changed repeatedly (Momeni et al. 1992).
Experiences on treatment of dermal injuries from SM poisoning have shown that even extremely extensive skin damage can be cured if the interfering of the infection is eliminated. Thus, the proper use of antibiotics is a critical step in management of the SM poisoning. Below some important drugs are tabulated (Balali-Mood et al. 2008; Vojvodic et al. 1985; Dabney et al. 1991). Different medications that were used for SM treatment is summarized in Table 4.2.
Table 4.2
Different pharmaceuticals were used to treat SM induced injuries
Class of drug | Drug name |
|---|---|
SM scavengers | Sodium thiosulphate, N-acetyl-L-cysteine, 4-Methyl-2-mercaptopyridine-1-oxide, Dimercaprol |
Anti-oxidants and inhibitors of lipid peroxidation | Vitamin-A, Vitamin-C, Vitamin-E, Hydroxyethyl, Rutoside |
Anti-inflammatory | Octyl homovanillamide, Indomethacin |
Protease inhibitor | 1-(40-Aminophenyl)-3-(4-chlorophenyl) urea, N-(0-P)-L-Ala-L-Ala-benzy ester hydrate |
(b)
Respiratory system
Management of SM-induced pulmonary injuries mainly include conservative and protective treatments such as inhalation corticosteroids therapy. Because oxidative stress and chronic inflammation is considered as the causality of lung injury, every therapeutic agent such as N-Acetyl Cysteine (NAC) that increase cellular anti-oxidant supply may be beneficial for treating lung injuries. Recently it is shown that nebulized morphine has beneficial effects on dyspnea, cough, respiratory rate, and heart rate in patients exposed to mustard gas (Shohrati et al. 2012).
4.8.1 New Therapeutic Approaches
4.8.1.1 Corticosteroids
Corticosteroids as a type of anti-inflammatory drugs are used in the treatment of patients with SM-induced respiratory illnesses such as asthma or chronic obstructive pulmonary disease. This steroid is usually applied in combination with non-steroidal anti-inflammatory drugs and antibiotics as a protective therapy. Studies show that this method of therapy ameliorate SM-induced skin injury (Dachir et al. 2004). Also, it is reported that corticosteroid inhalers specially fluticasone in combination with long-acting beta 2-agonists (salmetrol) may be effective in treatment of patients with chronic bronchiolitis (Ghanei et al. 2007).
4.8.1.2 Roxithromycin
Roxithromycin is a macrolide antibiotic that is typically used to treat respiratory and urinary tract and skin infections. Recently, it is shown that roxithromycin may have inhibitory effects on the cytotoxicity and inflammation in human bronchial and tracheal epithelial cells caused by exposure to SM (Gao et al. 2007). Also, roxithromycin significantly decrease the expression of pro-inflammatory cytokines including IL-1β, IL-6, IL-8 and TNF at both the protein and the mRNA level. Therefore, down-regulation of pro-inflammatory cytokines at DNA and protein level may play an important role in designing new therapeutic agents (Weinberger et al. 2011; Gao et al. 2007).
4.8.1.3 Iodine
Studies on an animal model showed that some agent such as iodine/povidone may be a potential antidote for the skin in SM induced poisoning (Wormser 1991). Treatment with provodine iodine (PI) may also protect from ulceration and vesication (Wormser et al. 1997). Other therapies for SM induced skin damage include glucose-saline treatment and sodium thiosulphate (Sugendran et al. 2013).
4.8.1.4 Recombinant Protein Technology
Recombinant DNA and protein technology may open a gate to design and develop new drugs in the future. For example, animal studies showed that recombinant human erythropoietin (rhEPO) and recombinant human granulocyte colony stimulating factor (rhG-CSF) stimulate the growth of the erythrocyte, reticulocyte and leucocyte (Cai et al. 2004). Keratinocyte suspension and stem cell technology may also develop and be used in epidermal wound healing of SM poisoning (Schmidt et al. 2013; Henemyre-Harris et al. 2008).
4.8.1.5 Epigenetic and Gene Therapy
Because, most of SM-induced toxicity is mediated through DNA damage; hence, epigenetic and gene therapy may be a new and valuable treatment modality in the treatment of SM poisoning. Epigenetic drugs may be used alone or in combination with other conventional drugs including antioxidants and anti-inflammatory agents to treat both acute and delayed SM-induced toxicity (Kunak et al. 2012).
4.8.1.6 Biologically Active Peptides
Antimicrobial peptides (AMPs) are novel type of antibiotics, which are thought to have key role in designing antibiotics and anti-inflammatory drugs in the near future (Asoodeh et al. 2012). Because a number of unusual peptides have been found in the blood of animals that treated with iodine, it is deduced that biologically active peptides may play critical role in the treatment of infection, and inflammation and disorders caused by oxidative stress (Brodsky et al. 2008). Also AMPs are a major part of the innate immune defense at the ocular surface and protect the epithelia of cornea and conjunctiva from microbial invasion (Garreis et al. 2010). AMPs as new sources of antibacterial are also involved in healing processes of the skin disease such as psoriasis, burns and wounds (Sorensen et al. 2003). Many antibacterial and anti-inflammatory and regulatory peptides have been reported so far, have good potential as antibacterial agents (Asoodeh et al. 2014). Recent studies suggest that peptide-drug conjugation may enhance the efficacy of drugs through different mechanistic pathways such as decreasing drug efflux via biological pumps (Fonseca and Kelley 2011).
4.8.1.7 Cell Death Suppressing
New therapeutic strategy may also rely on the preventing cell death and therefore vesication by inhibiting apoptosis with suppressing the death receptor (DR) or by administration of reducing biomolecules such as Niacinamide, Nicotinamide and Glutathione as potential inhibitors of cell death and promoters of cell survival (Keyser et al. 2014). A study showed that the SM-induced apoptosis pathway is via the Fas response; hence, caspase-3 activation can be inhibited by FasR siRNA and FasR antagonistic antibody (Keyser et al. 2013).
4.8.1.8 Herbal Medicine
Curcumin as medicinal herb is the yellow bioactive ingredient of Curcuma longa, which is shown to improve SM-induced chronic pruritus in Iranian patients (Panahi et al. 2012a). Pruritus is the most common chronic skin complication of mustard gas. This bioactive compound has anti-inflammatory effects and can reduce serum concentrations of interleukins (IL) 6 and 8 (Panahi et al. 2012b). Therefore, it is thought that curcumin may be a potential drug to treat cutaneous complications. Moreover, it is shown that Nigella sativa has protective effect lung inflammation in guinea pigs exposed to SM, it has also effects on tracheal responsiveness (Hossein et al. 2008).
4.9 Conclusion
Acute, chronic and delayed toxic effects of SM have been extensively studied, particularly on Iranian veterans. Despite the large number of studies, the mechanistic pathways of SM intoxication in cellular level, as well as clinical pharmacology and toxicology of MCs are less understood. In this chapter, toxic effects of mustard gas on different organs, particularly skin, lung, eyes and kidney have been comprehensively reviewed. In addition, long-term hematological complication, neurological impairment, and other delayed immunotoxicity have been discussed. Data shows that, SM-induced pulmonary toxicity, ocular irritation, and skin disease are the most affected organs of SM poisoning. The most important and plausible mechanistic pathways of SM intoxication include DNA alkylation, inflammatory response, ATP depletion, and oxidative stress.
It is recommended to conduct comprehensive studies both in vitro and in vivo to elucidate the exact mechanism of SM induced toxicity and to find new therapeutic modalities by using most recent technology.
Glossary
Alkylating agent
A molecule that transfer an alkyl group to target molecule, which acts as anticancer drugs by inhibiting DNA replication and transcription.
Biotransformation
Chemical modification of a compound in the body of living organism.
Bronchiectasis
A pulmonary disease in which some parts of lung airways is enlarged mainly due to inflammation or infection.
Calmodulin
A calcium binding protein in eukaryotic cells that regulates biological activities of calcium dependent proteins in many cellular processes.
Chemical warfare agent (CWA)
A chemically toxic substance that are used as chemical weapons in battlefield.
Corneal conjunctivalization
The presence of goblet cells in the corneal epithelium.
Epigenetic therapy




The use of drugs or epigenome-influencing techniques to treat diseases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


