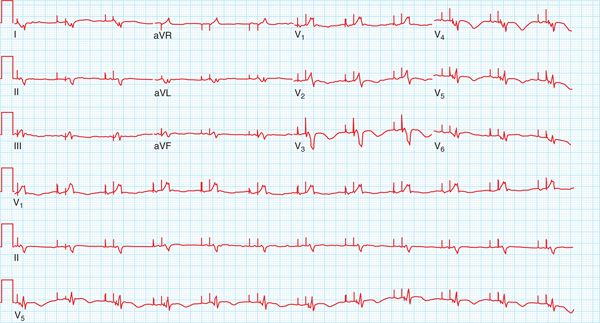
FIGURE 30-1 Twelve-lead electrocardiogram showing atrial flutter with 2:1 conduction. The corrected QT interval is 490 msec in the presence of a complete left bundle branch block.
FIGURE 30-2 Twelve-lead electrocardiogram obtained after two doses of dofetilide and spontaneous restoration of an AV sequentially paced rhythm. The corrected QT interval is 650 msec in the presence of biventricular pacing.
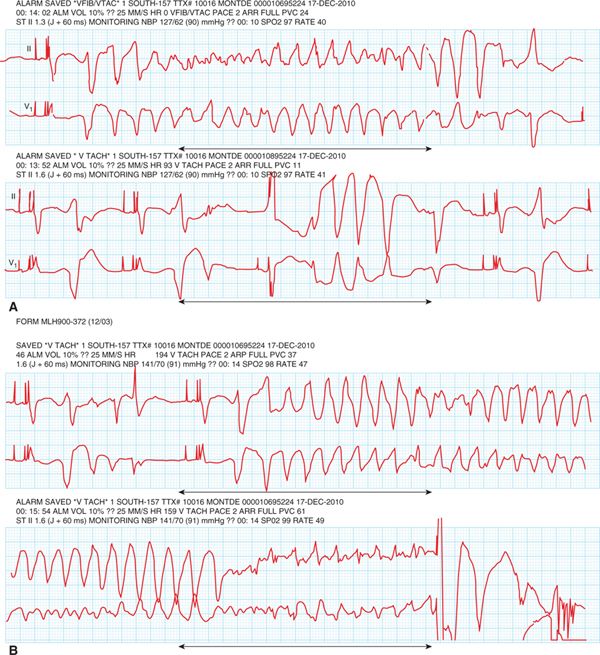
FIGURE 30-3 Telemetry strips showing polymorphic ventricular tachycardia in association with marked QT prolongation (torsade de pointes). Top strips (A) show nonsustained episodes of TdP and the bottom strip (B) shows an episode of TdP degenerating into ventricular fibrillation, terminated by an ICD discharge.
BACKGROUND
Class III Antiarrhythmic Agents
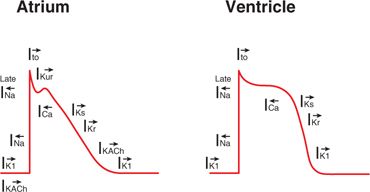
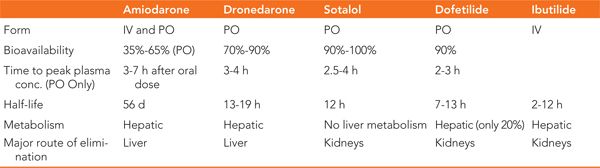
Vaughan-Williams class III drugs are membrane active antiarrhythmic agents that affect the potassium channels located on the surface of myocytes during the plateau and repolarization phases of the action potential (Figure 30-4). The duration of these phases are a direct result of the balance between the inward calcium currents and the outward potassium currents. Blocking of the potassium currents during repolarization causes a prolongation in the action potential duration with a resultant increase in the QT interval on the surface ECG. The most commonly used agents in this class are amiodarone, dronedarone, sotalol, dofetilide, and ibutilide. In addition to their predominant potassium channel effect, some of these drugs exhibit effects on other channels and/or receptors (Table 30-1). A comparison of their pharmacokinetic profiles is shown in Table 30-2.
FIGURE 30-4 Atrial and ventricular action potentials are shown along with their major ionic currents. Arrows denote direction of current (left = inward current; right = outward current). Class III antiarrhythmic agents block the potassium ion currents I(K).
TABLE 30-1 Class III Antiarrhythmic Channel/Receptor Effects
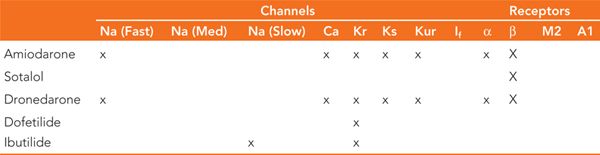
TABLE 30-2 Comparison of the Pharmacokinetics of Class III Antiarrhythmic Drugs
Class III Antiarrhythmic Drugs—General Indications and Usage
• All class III agents can be used for conversion and/or maintenance of sinus rhythm in patients with atrial fibrillation and atrial flutter.
• Amiodarone and sotalol also have indications in the treatment and prevention of ventricular arrhythmias.
• Drugs with predominantly class III effects (dofetilide, sotalol, ibutilide) may decrease defibrillation thresholds and can be used to facilitate direct-current cardioversions.
Proarrhythmic Effects of Class III Antiarrhythmic Agents
• The major side effect of this class of drugs is the risk of torsades de pointes (TdP), a type of polymorphic ventricular tachycardia associated with prolongation of the QT interval.
• Class III agents with predominant potassium channel blocking activity (sotalol, ibutilide, dofetilide) are more likely to cause TdP than those agents with multiple channel blocking activity (amiodarone, dronedarone).
• Patient factors that increase the risk of TdP include hypokalemia, hypomagnesemia, bradycardia, severe left ventricular dysfunction, impaired drug metabolism, and/or elimination and coadministration with other QT prolonging medications.
• Sotalol, dofetilide, and ibutilide demonstrate reverse-use dependence, resulting in a more potent potassium channel blocking effect at slower heart rates. This results in a longer QT interval with bradycardia and a higher risk of TdP.1
SPECIFIC AGENTS
Dofetilide
Dofetilide is a highly specific blocker of the rapid component of the delayed rectifier potassium current (Ikr), causing prolongation of the atrial and ventricular effective refractory periods. The drug is cleared predominantly through renal excretion and is contraindicated in patients with a creatinine clearance (CrCl) less than 20 mL/min. There are several drugs that significantly increase the plasma level of dofetilide and are contraindicated in its use. These include verapamil and trimethoprim, which increase the peak plasma concentrations of dofetilide, cimetidine, which inhibits renal tubular secretion of dofetilide, and ketoconazole, which is a significant inhibitor of the cytochrome p450 CYP3A4 isoenzyme, used in the hepatic metabolism of dofetilide. Hydrochlorothiazide is also contraindicated with the use of dofetilide due to both an increase in dofetilide’s serum plasma concentration and a reduction in serum potassium, the combination of which can result in a marked increase in the QT interval.
Indications
Dofetilide is currently indicated for the treatment of atrial fibrillation. In the SAFIRE-D trial,2 up to 29% of patients with persistent atrial fibrillation converted to sinus rhythm, most within the first 36 hours, and up to 58% of patients were able to maintain sinus rhythm for 1 year. In another study assessing the acute efficacy of dofetilide, Cotiga et al reported cardioversion rates ranging from 20% to 85% depending on the CrCl-based dosage.3 The risk of proarrhythmia, including TdP, in this same trial was 1.2%.
Current guidelines recommend dofetilide as a second line agent in patients with atrial fibrillation and no structural heart disease.4 In patients with depressed left ventricular function and congestive heart failure, however, dofetilide is a first-line agent as it has been shown to have a neutral effect on mortality in this patient population.5,6 Like other predominant potassium channel blockers, however, the use of dofetilide should be avoided in patients with significant hypertrophic cardiomyopathy due to an increased risk of proarrhythmia. While dofetilide is not indicated for use in patients with ventricular arrhythmias, there have been studies demonstrating a decreased incidence of ventricular tachycardia in patients with previously implanted cardioverter defibrillators.7
Dosing
• Dosing algorithm for dofetilide is shown in Table 30-3.
TABLE 30-3 Initial Dosing Schedule for Dofetilide
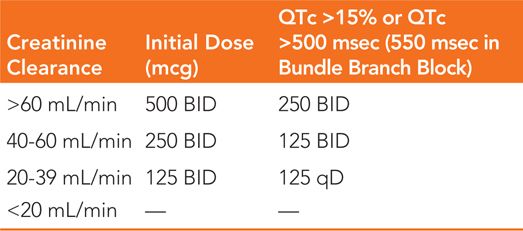
• Dofetilide should be discontinued if any QTc interval after the second or subsequent doses exceed 500 msec (550 msec in the presence of a bundle branch block).
• Requires in-hospital initiation.
• Correction of serum potassium and magnesium levels prior to drug initiation.
• Restricted to providers who have completed specialized training.
Side Effects
• No significant hemodynamic effects.
• Major cardiovascular adverse events are related to QT prolongation and risk of TdP (about 1%-3%).
• Risk factors for TdP include high dose, female gender, baseline QT >450 msec, and history of sustained VT.
• Excessive QTc increase during loading calls for immediate discontinuation of the drug.
• Avoid in combination with other QT prolonging agents (antifungals, macrolide antibiotics, and protease inhibitors).
Sotalol
Sotalol is a water-soluble, racemic mixture of L- and D-isomers. While both isomers have class III antiarrhythmic properties, the L-isomer also has mild β-blocker properties. Sotalol prolongs atrial and ventricular repolarization by blocking the rapid component of the Ikr current. ECG changes associated with the use of sotalol result from its β-blocking properties (slowing of the sinus rate and PR prolongation), as well as its class III potassium blocking activity (QT prolongation) in a dose-dependent fashion.
Indications
Sotalol is predominantly used for the treatment of atrial fibrillation and is considered a first-line antiarrhythmic agent in patients without structural heart disease or those with coronary artery disease and normal left ventricular function.4 It should be avoided in patients with severe left ventricular hypertrophy and also in those with left ventricular dysfunction. While not particularly effective in pharmacologic cardioversion, it is useful in the prevention of recurrent atrial fibrillation, especially at doses of 120 mg or 160 mg BID.8 Sotalol has also been shown to decrease the recurrence of ventricular arrhythmias in patients with inducible VT and also to decrease the incidence of ICD shock therapy in patients with a previously implanted cardioverter-defibrillator.9
Dosing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree