| Pain | An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage |
| Acute pain | Pain that is of short duration and resolves; usually directly related to the resolution or healing of tissue damage |
| Chronic pain | Pain that persists for longer than would be expected; an artificial threshold for chronicity (e.g., 1 month) is not appropriate |
| Neuropathic pain | Pain that arises from damage to, or dysfunction of, any part of the peripheral or central nervous system |
| Nociception | The process by which noxious stimuli produce activity in the sensory pathways that convey “painful” information |
| Allodynia | Pain caused by a stimulus that does not normally provoke pain |
| Hyperalgesia | An increased response to a stimulus that is normally painful |
| Analgesia | Any process that reduces the sensation of pain, while not affecting normal touch |
| Local anesthesia | Blockade of all sensation (innocuous and painful) from a local area |
| Noxious stimulus | Stimulus that inflicts damage, or would potentially inflict damage, on tissues of the body |
| Primary afferent neuron (PAN) | The first neuron in the somatosensory pathway; detects mechanical, thermal, or chemical stimuli at its peripheral terminals and transmits action potentials to its central terminals in the spinal cord; all PANs have a cell body in the dorsal root ganglion |
| Nociceptor | A primary afferent (sensory) neuron that is only activated by a noxious stimulus |
| Nociception | The process by which a nociceptor detects a noxious stimulus and generates a signal (action potentials) that is propagated towards higher centers in the nociceptive pathway |
| Dorsal root ganglion (DRG) | Contains the cell bodies of primary afferent neurons; proteins, including transmitters, receptors, and structural proteins, are synthesized here and transported to peripheral and central terminals |
| Interneuron | Neuron with its cell body, axon and dendrites within the spinal cord; can be excitatory (e.g., containing glutamate) or inhibitory (e.g., containing GABA) |
| Projection neurons | Neuron in the dorsal horn that receives input from PANs and/or interneurons, and projects up the spinal cord to higher processing centers |
| Spinothalamic tract | Tract of neurons that project from the spinal cord to the thalamus |
| Spinobulbar tracts | Several different tracts of neurons that project from the spinal cord to brainstem nuclei |
| Somatosensory cortex | Region of the cerebral cortex that receives input mainly from cutaneous sensory nerves; the cortex is topographically arranged, with adjacent areas receiving input from adjacent body areas; stimulation of the somatosensory cortex creates sensations from the body part that projects to it |
Many other chronic pain conditions may start centrally and never have a peripheral causation to the pain, especially conditions associated with multiple unexplained painful physical symptoms such as depression, anxiety, and fibromyalgia. Because these centrally mediated pain conditions are associated with emotional symptoms, this type of pain has until recently often been considered not to be “real” but rather a nonspecific outcome of unresolved psychological conflicts that would improve when the associated psychiatric condition improved; therefore, there was not a perceived need to target this type of pain. Today, however, many painful conditions without identifiable peripheral lesions and that were once linked only to psychiatric disorders are now hypothesized to be forms of chronic neuropathic pain syndromes that can be successfully treated with the same agents that treat neuropathic pain syndromes not associated with psychiatric disorders. These treatments include the SNRIs (serotonin–norepinephrine reuptake inhibitors: discussed in Chapter 7 on antidepressants) and the α2δ ligands (anticonvulsants that block voltage-gated calcium channels or VSCCs: discussed in Chapter 8 on mood stabilizers and in Chapter 9 on anxiety disorders). Additional psychotropic agents acting centrally at various other sites are also used to treat a variety of chronic pain conditions and will be mentioned below. Many additional drugs are being tested as potential novel pain treatments as well.
Since pain is clearly associated with some psychiatric disorders, and psychotropic drugs that treat various psychiatric conditions are also effective for a wide variety of pain conditions, the detection, quantification, and treatment of pain are rapidly becoming standardized parts of a psychiatric evaluation. Modern psychopharmacologists increasingly consider pain to be a psychiatric “vital sign,” thus requiring routine evaluation and symptomatic treatment. In fact, elimination of pain is increasingly recognized as necessary in order to have full symptomatic remission not only of chronic pain conditions, but also of many psychiatric disorders.
“Normal” pain and the activation of nociceptive nerve fibers
The nociceptive pain pathway is the series of neurons that begins with detection of a noxious stimulus and ends with the subjective perception of pain. This so-called nociceptive pathway starts from the periphery, enters the spinal cord, and projects to the brain (Figure 10-1). It is important to understand the processes by which incoming information can be modulated to increase or decrease the perception of pain associated with a given stimulus, because these processes can explain not only why maladaptive pain states arise but also why drugs that work in psychiatric conditions such as depression and anxiety can also be effective in reducing pain.

Figure 10-1. Activation of nociceptive nerve fibers. Detection of a noxious stimulus occurs at the peripheral terminals of primary afferent neurons and leads to generation of action potentials that propagate along the axon to the central terminals. Aβ fibers respond only to non-noxious stimuli, Aδ fibers respond to noxious mechanical stimuli and subnoxious thermal stimuli, and C fibers respond only to noxious mechanical, heat, and chemical stimuli. Primary afferent neurons have their cell bodies in the dorsal root ganglion and send terminals into that spinal cord segment as well as sending less dense collaterals up the spinal cord for a short distance. Primary afferent neurons synapse onto several different classes of dorsal horn projection neurons (PN), which project via different tracts to higher centers.
Nociceptive pathway to the spinal cord
Primary afferent neurons detect sensory inputs including pain (Figure 10-1). They have their cell bodies in the dorsal root ganglia located along the spinal column outside of the CNS and thus are considered peripheral and not central neurons (Figure 10-1). Nociception begins with transduction – the process by which specialized membrane proteins located on the peripheral projections of these neurons detect a stimulus and generate a voltage change at their peripheral neuronal membranes. A sufficiently strong stimulus will lower the voltage at the membrane (i.e., depolarize the membrane) enough to activate voltage-sensitive sodium channels (VSSCs) and trigger an action potential that will be propagated along the length of the axon to the central terminals of the neuron in the spinal cord (Figure 10-1). VSSCs are introduced in Chapter 3 and illustrated in Figures 3-19 and 3-20. Nociceptive impulse flow from primary afferent neurons into the CNS can be reduced or stopped when VSSCs are blocked by peripherally administered local anesthetics such as lidocaine.
The specific response characteristics of primary afferent neurons are determined by the specific receptors and channels expressed by that neuron in the periphery (Figure 10-1). For example, primary afferent neurons that express a stretch-activated ion channel are mechanosensitive; those that express the vanillinoid receptor 1 (VR1) ion channel are activated by capsaicin, the pungent ingredient in chili peppers, and also by noxious heat, leading to the burning sensation both these stimuli evoke. These functional response properties are used to classify primary afferent neurons into three types: Aβ, Aδ, and C-fiber neurons (Figure 10-1). Aβ fibers detect small movements, light touch, hair movement, and vibrations; C-fiber peripheral terminals are bare nerve endings that are only activated by noxious mechanical, thermal, or chemical stimuli; Aδ fibers fall somewhere in between, sensing noxious mechanical stimuli and subnoxious thermal stimuli (Figure 10-1). Nociceptive input and pain can thus be caused by activating primary afferent neurons peripherally, such as from a sprained ankle or a tooth extraction. NSAIDs (nonsteroidal anti-inflammatory drugs) can reduce painful input from these primary afferent neurons, presumably via their peripheral actions. Opioids can also reduce such pain, but from central actions, as explained below.
Nociceptive pathway from the spinal cord to the brain
The central terminals of peripheral nociceptive neurons synapse in the dorsal horn of the spinal cord onto the next cells in the pathway – dorsal horn neurons, which receive input from many primary afferent neurons and then project to higher centers (Figure 10-3). For this reason, they are sometimes also called dorsal horn projection neurons (PN in Figures 10-1, 10-2, and 10-3). Dorsal horn neurons are thus the first neurons of the nociceptive pathway that are located entirely within the CNS, and are therefore a key site for modulation of nociceptive neuronal activity as it comes into the CNS. A vast number of neurotransmitters have been identified in the dorsal horn, some of which are shown in Figure 10-2.

Figure 10-2. Multiple neurotransmitters modulate pain processing in the spinal cord. There are many neurotransmitters and their corresponding receptors in the dorsal horn. Neurotransmitters in the dorsal horn may be released by primary afferent neurons, by descending regulatory neurons, by dorsal horn projection neurons (PN) and by interneurons. Neurotransmitters present in the dorsal horn that have been best studied in terms of pain transmission include substance P (NK1, 2, and 3 receptors), endorphins (µ-opioid receptors), norepinephrine (α2-adrenergic receptors), and serotonin (5HT1B/D and 5HT3 receptors). Several other neurotransmitters are also represented, including VIP (vasopressin inhibitory protein and its receptor VIPR); somatostatin and its receptor SR; calcitonin-gene-related peptide (CGRP and its receptor CGRP-R); GABA and its receptors GABAA and GABAB; glutamate and its receptors AMPA-R (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor) and NMDA-R (N-methyl-D-aspartate receptor); nitric oxide (NO); cholecystokinin (CCK and its receptors CCK-A and CCK-B); and glycine and its receptor NMDA-R.
Neurotransmitters in the dorsal horn are synthesized not only by primary afferent neurons, but by the other neurons in the dorsal horn as well, including descending neurons and various interneurons (Figure 10-2). Some neurotransmitter systems in the dorsal horn are successfully targeted by known pain-relieving drugs, especially opioids, serotonin- and norepinephrine-boosting SNRIs (serotonin–norepinephrine reuptake inhibitors), and α2δ ligands acting at voltage-sensitive calcium channels (VSCCs). All of the neurotransmitter systems acting in the dorsal horn are potential targets for novel pain-relieving drugs (Figure 10-2), and a plethora of such novel agents is currently in clinical and preclinical development.
There are several classes of dorsal horn neurons: some receive input directly from primary sensory neurons, some are interneurons, and some project up the spinal cord to higher centers (Figure 10-3). There are several different tracts in which these projection neurons can ascend, which can be crudely divided into two functions: the sensory/discriminatory pathway and the emotional/motivational pathway (Figure 10-3).
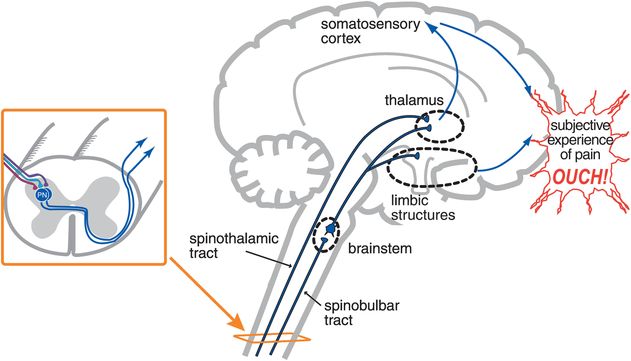
Figure 10-3. From nociception to pain. Dorsal horn neurons in the spinothalamic tract project to the thalamus and then to the primary somatosensory cortex. This pathway carries information about the intensity and location of the painful stimuli and is termed the discriminatory pathway. Neurons ascending in the spinobulbar tract project to brainstem nuclei and then to both the thalamus and limbic structures. These pathways convey the emotional and motivational aspects of the pain experience. Only when information from the discriminatory (thalamocortical) and emotional/motivational (limbic) pathways combine is the human subjective experience of pain formed (“ouch”).
In the sensory/discriminatory pathway, dorsal horn neurons ascend in the spinothalamic tract; then, thalamic neurons project to the primary somatosensory cortex (Figure 10-3). This particular pain pathway is thought to convey the precise location of the nociceptive stimulus and its intensity. In the emotional/motivational pathway, other dorsal horn neurons project to brainstem nuclei, and from there to limbic regions (Figure 10-3). This second pain pathway is thought to convey the affective component that nociceptive stimuli evoke. Only when these two aspects of sensory discrimination and emotions come together and the final, subjective perception of pain is created can we use the word pain to describe the modality (“ouch” in Figure 10-3). Before this point, we are simply discussing activity in neural pathways, which should be described as noxious-evoked or nociceptive neuronal activity but not necessarily as pain.
Neuropathic pain
The term neuropathic pain describes pain that arises from damage to, or dysfunction of, any part of the peripheral or central nervous system, whereas “normal” pain (so-called nociceptive pain, just discussed in the section above) is caused by activation of nociceptive nerve fibers.
Peripheral mechanisms in neuropathic pain
Normal transduction and conduction in peripheral afferent neurons can be hijacked in certain neuropathic pain states to maintain nociceptive signaling in the absence of a relevant noxious stimulus. Neuronal damage by disease or trauma can alter electrical activity of neurons, allow cross-talk between neurons, and initiate inflammatory processes to cause peripheral sensitization. In this chapter, we will not emphasize peripheral sensitization disorders and mechanisms, but rather central sensitization disorders and mechanisms.
Central mechanisms in neuropathic pain
At each major relay point in the pain pathway (Figure 10-3), the nociceptive pain signal is susceptible to modulation by endogenous processes to either dampen down the signal or amplify it. This happens not only peripherally at primary afferent neurons, as has just been discussed, but also at central neurons in the dorsal horn of the spinal cord as well as in numerous brain regions. The events in the dorsal horn of the spinal cord are better understood than those in brain regions of nociceptive pathways, but pain processing in the brain may be the key to understanding the generation and amplification of central pain in disorders of chronic peripheral pain such as osteoarthritis, low back pain, and diabetic peripheral neuropathic pain, as well as painful physical symptoms in affective and anxiety disorders and in fibromyalgia.
“Segmental” central sensitization is a process thought to be caused when plastic changes occur in the dorsal horn, classically in conditions such as phantom pain after limb amputation. Specifically, this type of neuronal plasticity in the dorsal horn is called activity-dependent or use-dependent, because it requires constant firing of the pain pathway in the dorsal horn. The consequence of this constant input of pain is eventually to cause exaggerated (hyperalgesic) or prolonged responses to any noxious input – a phenomenon sometimes called “wind-up” – as well as painful responses to normally innocuous inputs (called allodynia). Phosphorylation of key membrane receptors and channels in the dorsal horn appears to increase synaptic efficiency and thus to trip a master switch, opening the gate to the pain pathway and turning on central sensitization that acts to amplify or create the perception of pain even if there is no pain input coming from the periphery. The gate can also close, as conceptualized in the classic “gate theory” of pain, in order to explain how innocuous stimulation (e.g., acupuncture, vibration, rubbing) away from the site of an injury can close the pain gate and reduce the perception of the injury pain.
In segmental central sensitization, a definite peripheral injury (Figure 10-4A) is combined with central sensitization at the spinal cord segment receiving nociceptive input from the damaged area of the body (Figure 10-4B). Segmental central sensitization syndromes are thus “mixed” states where the insult of central segmental changes (Figure 10-4B) is added to peripheral injuries such as low back pain, diabetic peripheral neuropathic pain, and painful cutaneous eruptions of herpes zoster (shingles) (Figure 10-4A).
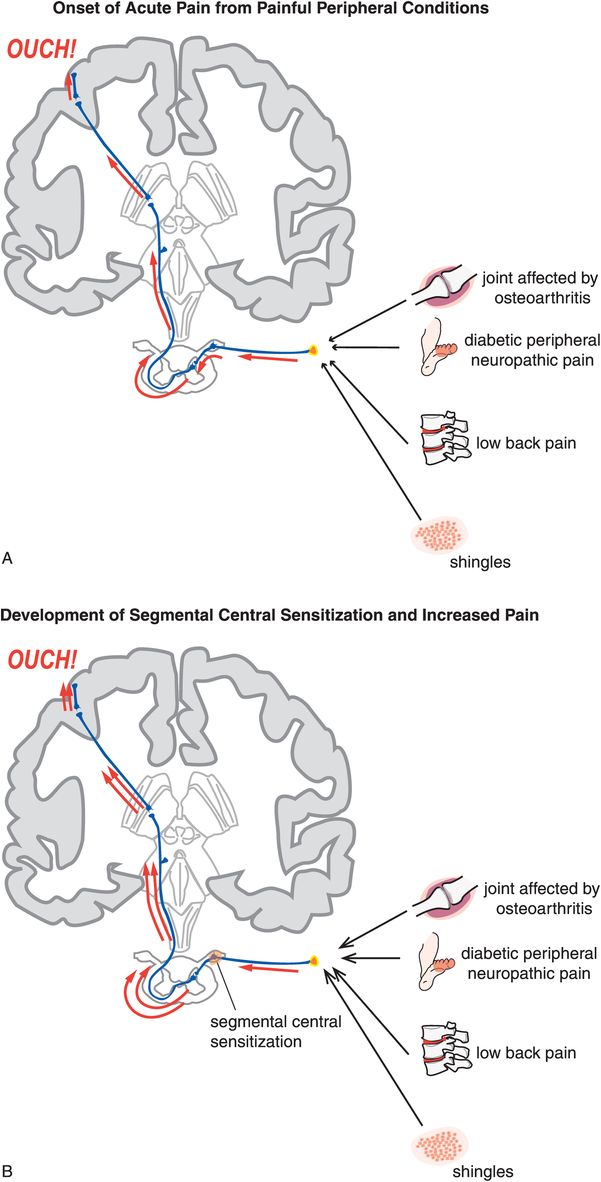
Figure 10-4. Acute pain and development of segmental central sensitization. (A) When peripheral injury occurs, nociceptive impulse flow from primary afferent neurons is transmitted via dorsal horn neurons to higher brain centers, where it can ultimately be interpreted as pain (represented by the “ouch”). (B) In some cases, injury or disease directly affecting the nervous system may result in plastic changes that lead to sensitization within the central nervous system, such that the experience of pain continues even after tissue damage is resolved. Impulses may be generated at abnormal locations either spontaneously or via mechanical forces. At the level of the spinal cord, this process is termed segmental central sensitization. This mechanism underlies conditions such as diabetic peripheral neuropathic pain and shingles.
“Suprasegmental” central sensitization is hypothesized to be linked to plastic changes that occur in brain sites within the nociceptive pathway, especially the thalamus and cortex, in the presence of known peripheral causes (Figure 10-5A) or even in the absence of identifiable triggering events (Figure 10-5B). In the case of peripherally activated suprasegmental central sensitization, it is as though the brain “learns” from its experience of pain, and decides not only to keep the process going, but also to enhance it and make it permanent. In the case of pain that originates centrally without peripheral input, it is as though the brain has figured out how to spontaneously activate its pain pathways. Interrupting this process of sensitized brain pathways for pain and getting the CNS to “forget” its molecular memories may be one of the greatest therapeutic opportunities in psychopharmacology today, not only because this may be a therapeutic strategy for various chronic neuropathic pain conditions, as discussed here, but also because it may be a viable approach to treating the hypothesized molecular changes that may underlie disease progression in a wide variety of disorders, from schizophrenia to stress-induced anxiety and affective disorders, to addictive disorders. Conditions hypothesized to be caused by suprasegmental central sensitization syndromes of pain originating in the brain without peripheral pain input include fibromyalgia, the syndrome of chronic widespread pain, and painful physical symptoms of depression and anxiety disorders, especially PTSD (Figure 10-5B).
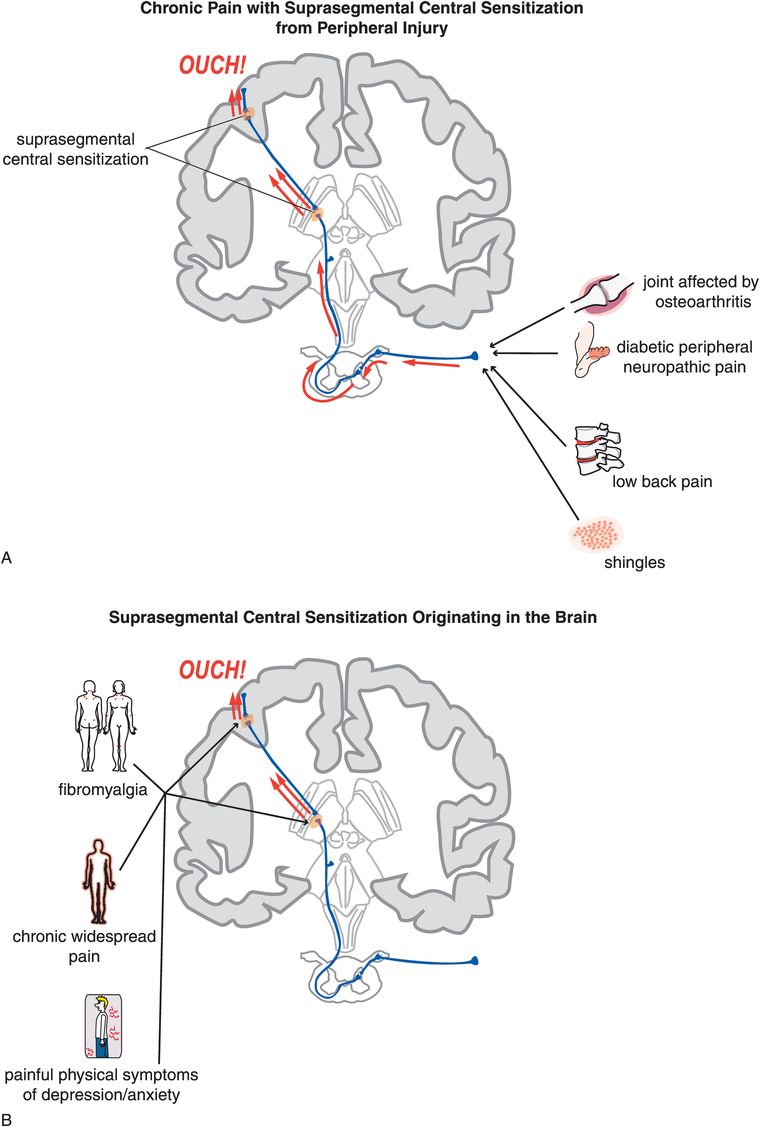
Figure 10-5. Suprasegmental central sensitization. Plastic changes in brain sites within the nociceptive pathway can cause sensitization, for instance at the level of the thalamus or the sensory cortex. This process within the brain is termed suprasegmental central sensitization. This can occur following peripheral injury (A) or even in the absence of identifiable triggering events (B). This mechanism is believed to underlie conditions such as fibromyalgia, chronic widespread pain, and painful symptoms in depression and anxiety disorders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


