CHAPTER 24 Chronic myelogenous leukemia
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm consistently associated with the BCR-ABL1 fusion gene (reviewed in1). CML is characterized by neutrophilic granulocytosis and pathologic left shift in the peripheral blood (PB) with less than 20% (WHO) or 30% (European LeukemiaNet) blasts in blood and bone marrow (BM).2,3 CML is a disease most frequently occurring in the middle age with a peak in the 5th and 6th decades of life, affecting about 1–2 persons/100 000.3 It may also occur in younger and older patients, but it is rarely seen in childhood.
Increased risk of CML had been observed in persons exposed to ionizing radiation.5 However, history of radiation exposure is unusual in patients with CML so that the exact cause of disease remains unclear in the great majority of patients.5 Inherited predisposition to CML has not been reported, but risk of CML appears to be reduced in persons with HLA-B3 and -B8 phenotype, indicating that some unknown predisposing factors may exist.6
Animal models7 and the success of BCR-ABL tyrosine kinase (TK) inhibitors in inducing a remission of CML8 have impressively demonstrated the relevance of the BCR-ABL1 fusion to the pathogenesis of CML (described in detail in Chapter 21). Monotherapy with BCR-ABL TK inhibitors induces a complete cytogenetic (CCyR) and a major molecular (MMR) remission in the great majority of patients.9,10
Clinical features and course of disease
CML shows a characteristic bi- or triphasic course, starting with a rather indolent chronic phase (CP) and transforming to a usually lethal final acute phase, a dramatic clinical picture called ‘blast crisis’ (Table 24.1). In more than 95% of patients, CML is diagnosed in CP. Rarely, CML is diagnosed in acute phase with a clinical picture of a BCR-ABL1-positive acute myeloid (AML) or lymphatic leukemia (ALL).
During the last 3 decades, the median duration of the CP could be markedly prolonged from less than 511 to more than 10 years.12 Before the use of TK inhibitors, the majority of patients developed blast crisis several years after diagnosis of CML.11,12 After diagnosis of blast crisis, patients die within few months, usually from fatal infection or bleeding or organic failure due to blast infiltration.13
In the majority of patients, blast phase is preceded by an accelerated phase characterized by therapy resistance with increasing leuko- or thrombocytosis, increasing counts of immature myeloid precursors in PB, progressive basophilia, splenomegaly or hepatomegaly, and reduced quality of life. From the time of diagnosis of accelerated phase, the survival time is markedly reduced (median less than 2 years).1
With the application of BCR-ABL specific TK inhibitors for therapy of CML, prognosis and quality of life of patients have dramatically improved since the risk of progression to accelerated or acute phase has been significantly reduced when compared to earlier therapy concepts.10,14
Diagnostic procedures
Genetics
Evidence of the Philadelphia chromosome or the reciprocal translocation t(9;22)(q34;q11.2) confirms the CML diagnosis3 in patients with characteristic morphologic changes in blood and BM (see below). Molecular evidence of the BCR-ABL1 fusion is mandatory in patients without the Philadelphia chromosome. Cytogenetic and molecular studies are also used to control the efficacy of treatment (see Chapter 21 for details). Nowadays, disease is considered therapy-resistant in patients who do not achieve cytogenetic remission2 but patients who achieve a continuing CyCR have an excellent prognosis.15
Whereas additional chromosomal changes besides t(9;22)(q34;q11.2) do not appear to provide independent prognostic information when occurring prior to anti-neoplastic therapy,16 clonal evolution may support diagnosis of therapy resistance or accelerated phase when occurring during the course of CML.17,18
BCR-ABL1 mutations are frequent in patients who become resistant to a therapy with a TK inhibitor.17,19 Therefore, molecular analysis of BCR-ABL1 mutations has become a further standard procedure in patients losing their cytogenetic or molecular response to therapy.2
Morphology
Morphological diagnosis of CML is based on characteristic changes in blood and BM (Table 24.1). Organomegaly (splenomegaly, hepatomegaly) may also be present at diagnosis, indicating extramedullary expansion of disease.16
At diagnosis
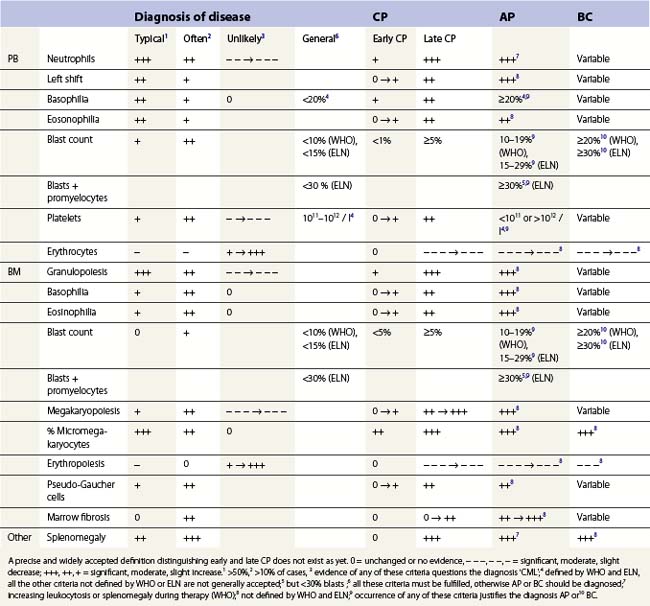
Peripheral blood. The first diagnostic feature usually arousing suspicion of CML is a leukemic PB picture. Granulocytosis with a pathologic left shift without hiatus leukaemicus is the main characteristic (Fig. 24.1). Granulocytosis comprises neutrophils, eosinophils and basophils. Myelocytes usually predominate among immature precursors mobilized into PB, but promyelocytes and myeloblasts may also occur especially in the late chronic or the accelerated phase (Fig. 24.1). The WHO classification defines a blast count of less than 20%, otherwise the disease should be classified as an acute leukemia.3 Some therapy trials use the previous FAB classification definition, where the count of blasts and promyelocytes should be less than 30%.2 Thrombocytosis is a common finding and when excessive (>106/l) may lead to the misdiagnosis of ‘essential thrombocythemia’ (ET).20,21 However, CML with thrombocytosis differ from ET by a pathologic left shift and basophilia in the differential count, characteristic morphologic changes in BM (see below), evidence of the BCR-ABL1 fusion, and in most cases, lack of the JAK2V617F mutation (see Chapters 21 and 23 for details).
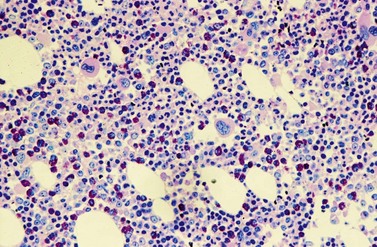
Changes in hematopoiesis. First obvious morphologic feature in microscopic evaluation at low magnification is a marked expansion of neutrophilic granulopoiesis (Fig. 24.2). In the majority of patients, eosinophilic granulopoiesis is also increased. Basophilic granulopoiesis is at least slightly increased in almost all patients (Fig. 24.3). In the majority of patients, granulopoiesis shows a complete maturation (Fig. 24.2), but it is often left shifted with a slight relative increase of myelocytes. In some patients, numbers of promyelocytes and myeloblasts are also increased. The myeloblast count is usually lower than 10%. The neoplastic granulocytes show a reduced leukocyte alkaline phosphatase (LAP) index, which distinguishes them from non-neoplastic granulocytes in leukemoid reactions.22 However, this does not significantly influence their function so that infections due to granulocytic dysfunction are uncommon in CP of CML. In the past, determination of the LAP index of granulocytes was a standard method applied at diagnosis of CML. Molecular investigation for the presence of the BCR-ABL1 fusion has replaced this procedure.
A further characteristic feature is an increase of micromegakaryocytes with a central hypolobulated nucleus, which is due to a reduction of mature hyperploid megakaryocytes23,24 (Figs 24.4 and 24.5). The exact mechanism leading to the lack of hyperploid megakaryocytes is not clear as yet. This feature is pathognomonic for CML and lack of it should question the diagnosis of CML. Some authors have divided CML according to the count of micromegakaryocytes into a megakaryocytic-rich and a granulocytic subtype.23,25 The megakaryocytic-rich subtype differs from the granulocytic by an increased risk of BM fibrosis.
Although erythropoiesis in CML is also BCR-ABL1 positive, it is usually not expanded. In the majority of patients, erythropoiesis is rather reduced, particularly in late chronic, accelerated or blastic phase, correlating with a varying degree of anemia.25
Except for micromegakaryocytes, dysplastic features of hematopoiesis are uncommon in CP of CML. Platelets may show dysfunction with increased risk of bleeding, but thromboembolic events are uncommon.26
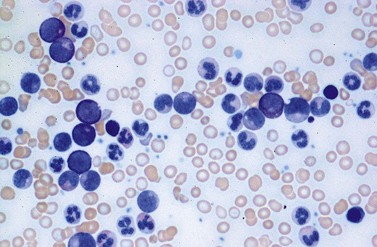
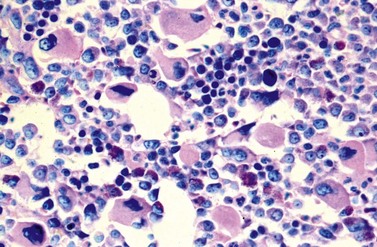
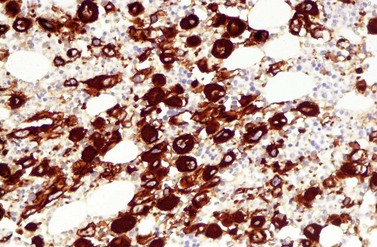
A blast count exceeding 10% of marrow cells is not common at diagnosis, but a typical feature during the course of CML, especially in accelerated or blastic phase (Fig. 24.7). Blasts occur mainly within the proliferation zone of granulopoiesis (myeloblasts; Fig. 24.7), but they may also be observed outside of it, particularly lymphoblasts or megakaryoblasts (Fig. 24.8). By immunohistochemistry, myeloblasts are often but not always CD34 or CD117 positive with co-expression of CD33, myeloperoxidase or lysozyme. Megakaryoblasts are CD42b or CD61, and lymphoblasts have B-precursor related phenotype: TdT, CD34, CD10, CD20 and CD79a positive. Flow cytometry may be useful by demonstrating aberrant CD56 expression in granulopoiesis, which is, however, also observed in other myeloproliferative and myelodysplastic disorders.27 This method can also help in early detection of incipient blast crisis and in differentiating between lymphatic and myeloid blastic transformation.28
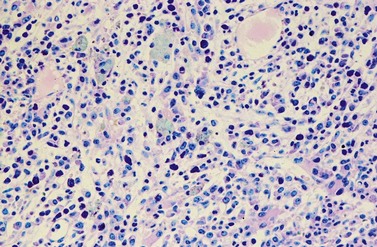
Changes of non-hematopoietic tissue. First obvious morphologic feature in microscopic evaluation at low magnification is the replacement of the adipose BM tissue usually to less than 5% of marrow volume (Figs 24.2, 24.5–7). A further typical feature is the occurrence of storage histiocytes resembling Gaucher cells, the so-called pseudo-Gaucher cells29 (Figs 24.2, 24.5). These histiocytes belong to the neoplastic clone,30 and their Gaucher-like appearance is caused by a relative enzyme insufficiency as a result of an excessive phagocytosis of leukemic cells whereas the mesenchymal stem cells, which differentiate into adipocytes, fibroblasts and osteoblasts are not involved in the neoplastic proliferation.31
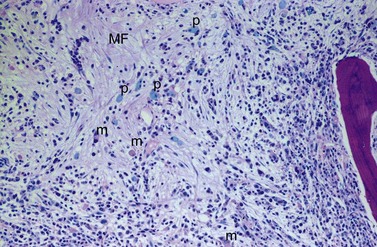
BM fibrosis is another typical feature of CML (Figs 24.5, 24.6).32 It affects only a minority of patients at diagnosis, but a significant proportion of patients develop fibrosis during the course of disease, especially when therapy has lost its efficacy.33,34 In patients not responding to therapy, the cumulative proportion of cases with BM fibrosis amounts to more than 90%.33 Therefore, CML is the second myeloproliferative neoplasm besides primary myelofibrosis (PMF) characterized by a high risk of BM fibrosis.
Marrow fibrosis usually starts focally (patchily), followed by transformation to diffuse extensive fibrosis developing during course of disease.34 As in PMF, the fiber-producing cells (fibroblasts) are not part of the neoplastic clone.31 However, their fiber production is up-regulated by growth factors produced by the neoplastic hematopoiesis, particularly platelet-derived growth factor and transforming growth factor-β, resulting in increased collagen type I and III (reticulin) fiber deposits.35
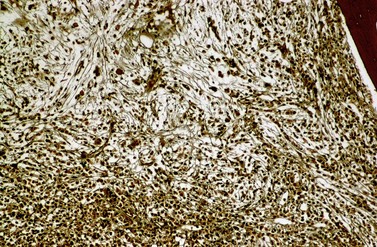
Fibrosis becomes visible after silver impregnation (Fig. 24.6). Various methods were presented with respect to grading of marrow fibrosis (see Chapter 3), but a worldwide uniform approach to diagnosis and quantification of marrow fibrosis does not exist so far. In general, more than 5% of marrow volume per µm thickness of tissue section with fiber deposits >104 mm/mm3 are uncommon in normal healthy subjects so that this limit can be applied to diagnose BM fibrosis.36 In fibrotic BM regions in CML, the density of the fiber deposits usually exceeds 104 mm/mm3.36
BM fibrosis correlates with an increased megakaryocyte count, splenomegaly and mobilization of normoblasts in PB.25 It may also correlate with an excess of blasts. Extensive diffuse fibrosis may be accompanied with osteosclerosis.
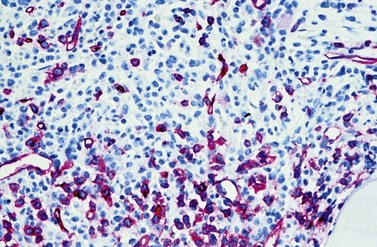
Another characteristic feature that CML shares with other neoplastic disorders is an increased BM vascularity37 (Fig. 24.7). It results from an increased production of pro-angiogenic growth factors by the neoplastic clone, especially the vascular endothelial growth factor A (VEGF). It has been found that BCR-ABL1 oncoprotein could in vitro induce VEGF promoter activity and increased VEGF protein levels in Ba/F3 cells as well as promote the expression of functionally active hypoxia-inducible factor-1 (HIF-1), a major transcriptional regulator of VEGF gene expression.38 Furthermore, the pluripotent neoplastic stem cell appears to be able to differentiate into endothelial cells.39 Thus, CML seems to be able to induce BM hypervascularity as a part of the neoplastic proliferation.
Extramedullary manifestations. Up to 70% of CML patients at diagnosis show a varying degree of hepato- and splenomegaly.16 Spleen enlargement results from an infiltration of red pulp cords by neoplastic hematopoiesis, mainly granulopoiesis. Hepatomegaly is a result of intrasinusoidal and periportal infiltration by hematopoiesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree