Chronic Myelogenous Leukemia, BCR-ABL1+
Kaaren K. Reichard, MD
Key Facts
Clinical Issues
Classically 3 phases of disease: Chronic, accelerated, and blast
Microscopic Pathology
Peripheral blood, chronic phase
Granulocytic leukocytosis with left shift and < 5% blasts
Basophilia
Bone marrow, chronic phase
Blasts < 5%
Granulocytic predominance with M:E ratio > 10
Small, monolobulated megakaryocytes
May see reticulin fibrosis
Ancillary Tests
Flow cytometry identifies blast lineage in accelerated and blast phases
Genetic studies required to confirm t(9;22) (q34;q11.2) or BCR-ABL1 fusion
Cytogenetic studies reveal clonal evolution portending disease progression
Molecular studies (BCR-ABL1 transcript levels) for minimal residual disease monitoring
Top Differential Diagnoses
Leukemoid reaction
BCR-ABL1(-) myeloproliferative neoplasm
Chronic neutrophilic leukemia
Myelodysplastic/myeloproliferative neoplasm
Atypical CML
TERMINOLOGY
Abbreviations
Chronic myelogenous leukemia (CML)
Synonyms
Chronic myeloid leukemia
Chronic granulocytic leukemia
Definitions
Myeloproliferative neoplasm that derives from clonal hematopoietic stem cell
Diagnosis rests on identification of t(9;22)(q34;q11.2) (or variant) or BCR-ABL1 genetic fusion
ETIOLOGY/PATHOGENESIS
Environmental Exposure
Radiation exposure implicated in some cases
Most predisposing factors unknown
Rare cases follow potent chemotherapy
BCR-ABL1 Fusion Protein
Using sensitive molecular techniques, very low levels of BCR-ABL1 fusion detected in healthy individuals
Individuals do not develop myeloid neoplasms
BCR-ABL1 fusion not specific for CML
Seen in some acute lymphoid and myeloid leukemias
Fusion transcript may be different
BCR-ABL1
Chronic phase
Consequence of BCR-ABL1 fusion is protein constitutive tyrosine kinase activity
Acquisition of preleukemic genetic abnormalities
Leads to proliferation and survival advantage
Results in myeloproliferation
Transformation to blast phase
Increased BCR-ABL1 transcript levels
Differentiation arrest
Expression of transcription factors normally involved in maturation are lost (e.g., CEPBA)
Additional chromosomal abnormalities: +8, duplicate Philadelphia chromosome, i(17q), +17, +19
Inactivation of tumor suppressor genes (e.g, TP53) results in unchecked cell division
Gene expression profiling shows numerous other recurrent genetic abnormalities in CML progression
CML Stem Cell
BCR-ABL1 transforms cell that has inherent selfrenewal capabilities
Supports concept of clonal hematopoietic stem cell disorder
Quiescent BCR-ABL1-expressing leukemic stem cells resistant to chemotherapy, radiation, and targeted tyrosine kinase inhibitors
Targeted therapy (e.g., imatinib) theorized to eliminate differentiated cells
Leukemic stem cells remain, leading to persistent residual disease
Cells produced in bone marrow (even lymphocytes) contain the BCR-ABL1 fusion
CLINICAL ISSUES
Epidemiology
Incidence
1-2 cases per 100,000 people per year
Age
Median at diagnosis is 50-70 years, but may occur at any age
Gender
Slight male predominance
Ethnicity
No reported ethnic predisposition
Presentation
Splenomegaly
Weight loss
Fatigue
Night sweats
Abnormal CBC
Leukocytosis
Basophilia
20-30% asymptomatic
Detected by routine CBC screening
Treatment
Drugs
Historically, nontargeted therapies utilized
Radiation, busulfan, hydroxyurea, interferon alpha
Varying degrees of activity
Currently, targeted therapies (tyrosine kinase inhibitors, a.k.a. TKIs)
1st generation TKI, imatinib mesylate (STI151)
Targets the abnormal BCR-ABL1 fusion kinase
Most data from the IRIS study (randomized clinical trial with > 5 years of follow-up)
Many patients respond to standard oral dose of imatinib (400 mg/day)
Some patients do not achieve adequate levels of response or discontinue therapy because of resistance; increasing dose of imatinib may help; response usually modest
Side effects of imatinib: Muscle cramps, edema, diarrhea, skin rash, myelosuppression
2nd generation TKIs
Nilotinib (10-30 fold increased potency in imatinib-resistant patients)
Dasatinib inhibits all imatinib-resistant BCR-ABL1 mutations, except T315I
Both of these drugs are FDA-approved for use in patients with imatinib resistance/intolerance
Both also show decent activity in patients with relapsed chronic phase CML
Allogeneic stem cell transplantation
Only proven curative therapy
Associated with significant morbidity and mortality
Prognosis
Prior to TKI targeted therapies, median survival ranged from 3-6 years
With TKIs, prognosis determined by rate of hematologic, cytogenetic, and molecular response
Imatinib 5-year overall survival is 80-95% (chronic phase)
Mechanisms of relapse/resistance
BCR-ABL1 kinase domain mutations
40-90% of patients resistant to imatinib have demonstrable mutation
Mutations that occur at contact point of imatinib and ABL1 kinase
Examples include T315I and F359V
T315I mutations are insensitive to 1st- and 2nd-line TKIs
Mutations that affect conformation of kinase; imatinib cannot bind
Mutations in P loop: M244V, E255K/V, etc.
Mutations in activation loop H396R/P
3 classically defined phases of disease: Chronic, accelerated, and blast
Chronic: < 5% blasts
Accelerated and blast phases
Represent disease progression
Generally refractory to therapy
Blast phase (≥ 20% blasts); 75-80% myeloid lineage, 20-25% lymphoid lineage (predominantly B)
Moderate/marked reticulin fibrosis in chronic phase associated with worse prognosis
Minimal residual disease (MRD) monitoring
MRD monitored using quantitative PCR (Q-PCR) for BCR-ABL1 transcript levels
> 3 log reduction within 1st 12-18 months of imatinib therapy predictive of long-term disease remission status
IMAGE FINDINGS
Radiographic Findings
Splenomegaly
MICROSCOPIC PATHOLOGY
Predominant Pattern/Injury Type
Hyperplasia
Predominant Cell/Compartment Type
Hematopoietic, myeloid
Key Microscopic Features
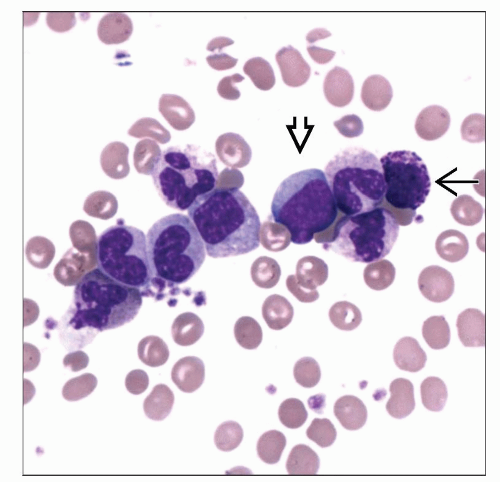
Peripheral blood (chronic phase)
Granulocytic leukocytosis with left shift to immaturity
Predominance of neutrophils and myelocytes
Blasts < 5%
Basophilia
Often eosinophilia
No or mild anemia
Preserved/elevated platelet count
Atypical large platelets or megakaryocytic cytoplasmic fragments
Circulating megakaryocytic nuclei
Circulating nucleated red blood cells
No significant granulocytic dysplasia
Bone marrow aspirate (chronic phase)
Hypercellular
Blasts < 5%
Granulocytic lineage predominates with myeloid:erythroid ratio > 10:1
Minimal dysplasia in any cell line
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree