18 Chronic kidney disease and end’stage renal disease
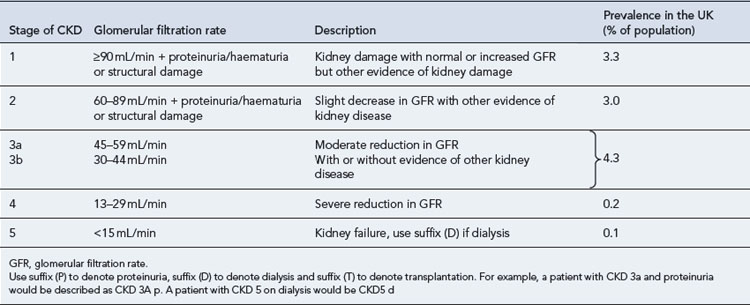
Chronic kidney disease (CKD) is defined by a reduction in the glomerular filtration rate (GFR) and/or urinary abnormalities or structural abnormalities of the renal tract. The severity of CKD is classified from 1 to 5 depending upon the level of GFR (Table 18.1). It is a common condition affecting up to 10% of the population in Western societies and is more common in some ethnic minority populations and in females. The incidence increases exponentially with age such that some degree of CKD is almost inevitable in persons over 80 years of age. Social deprivation is also associated with a higher prevalence of CKD. The scale of CKD and the consequences for the health service has been appreciated only in the last few years.
Estimates for the incidence of the various grades of CKD are shown in Table 18.1 and have been derived from large American studies, although data suggests the rates in the UK are similar (UK Renal Registry, 2008). In the past, patients with CKD were often unrecognised owing to difficulties in measuring or estimating the GFR and their health needs were largely unmet. The recent development of simple methods to estimate GFR has revealed a huge population of patients with significant kidney disease. This will pose a considerable challenge to health services in the future. National guidance on the management of CKD has been published (NICE, 2008) and includes management in primary and secondary care.
Renin-angiotensin-aldosterone system
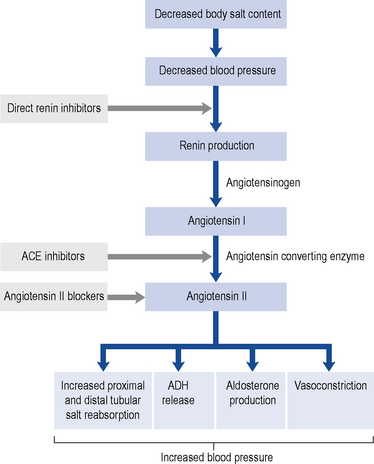
Renin promotes cleavage of the protein angiotensinogen, which is produced by the liver, to produce angiotensin I. Angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II has two major physiological effects. First, it acts on the zona glomerulosa of the adrenal cortex to promote production of the mineralocorticoid hormone aldosterone, with resultant increased distal tubular salt and water reabsorption. Furthermore, it promotes antidiuretic hormone (ADH) release, which increases proximal tubular sodium reabsorption and promotes thirst. In combination, these lead to salt and fluid retention, high intravascular volumes, hypertension and oedema. Second, it is a direct vasoconstrictor and promotes systemic and (preferential) renal hypertension. The renal effects are predominantly on the efferent glomerular arteriole. Vasoconstriction at this site is mediated by a high density of angiotensin II receptors. When these receptors are ligated by angiotensin II, there is increased intra-glomerular pressures. Whilst this leads to an overall increase in GFR in the short-term, over a longer period glomerular hypertension promotes accelerated glomerular scarring and worsening CKD. In addition to the vascular and endocrine effects of the RAAS, it is now recognised that there is a local immune modulatory role for this system. Both resident (e.g. tubular epithelial) cells and inflammatory (monocytes and macrophages) cells synthesise components of the RAAS and are themselves targeted by the system. For example, monocytes and macrophages express the angiotensin II receptor and activation through this receptor leads to an enhanced inflammatory and fibrotic phenotype of the cell. This raises the intriguing concept that some of the effects of blocking the RAAS are due to direct anti-inflammatory and anti-fibrotic effects. Figure 18.1 shows this pathway and identifies the points at which pharmacological interventions targeted for a biological effect translates into clinical outcomes.
Measurement of renal function
MDRD glomerular filtration rate equation
Eight eGFR equations were validated for the MDRD study (Levey et al., 1999). These used demographic and serum variables (including serum creatinine level, age, gender, non-black ethnicity, higher serum urea levels, and lower serum albumin levels) in a series of equations. The four-variable equation (also known as the abbreviated (a)MDRD equation) has been adopted into clinical practice and incorporates age, creatinine, gender and ethnicity (Fig. 18.2).
Other estimates of kidney function
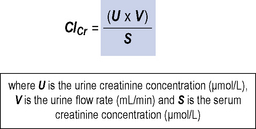
Creatinine clearance
This is similar to the GFR as nearly all the filtered creatinine appears in the urine. It is a measurement of the volume of blood that is cleared of creatinine with time. Measurements of creatinine clearance (ClCr) require accurate collection of 24 h urine samples with a serum creatinine sample midway through this period. This is time-consuming, inconvenient and prone to inaccuracy and as such is now rarely used in clinical practice. Figure 18.3 shows the equation for measuring creatinine clearance.
Cockroft–Gault equation
The Cockroft–Gault equation uses weight, sex and age to estimate creatinine clearance and was derived using average population data (Cockroft and Gault, 1976). The equation is shown in Fig. 18.4.
Estimates of glomerular filtration rate in paediatric patients
Estimates of GFRs in paediatric patients can be made using the Schwartz formula (Schwartz, 1985) or the Counahan–Barratt method (Counahan et al., 1976) which both rely upon inclusion of the height of the child in estimating creatinine clearance, since height correlates with muscle mass.
Significance of CKD
Patients with CKD 1–3 (Table 18.1) are frequently asymptomatic. The reduction of GFR is insufficient to cause uraemic symptoms and any minor abnormalities in the urine such as proteinuria or haematuria are usually not noticed by patients. There is a frequent association with high blood pressure which may be the cause or a consequence of renal damage. Recognition of these patients is important as it allows early modification of traditional cardiovascular risk factors. These patients should be investigated to determine if there is a treatable cause for their CKD and followed up to identify those individuals with progressive disease.
Patients with CKD stages 4 and 5 (Table 18.1) should usually be followed up in a nephrology clinic because they will require specialist management of the complications of CKD such as anaemia and bone disease, whilst many will also be undergoing preparation for renal replacement therapy.
Causes of CKD
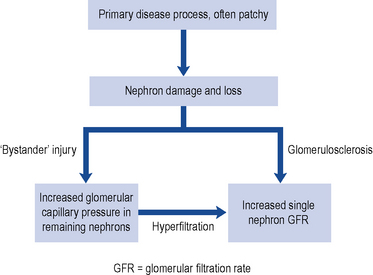
The reduction in renal function observed in CKD results from damage to the infrastructure of the kidney in discrete areas rather than throughout the kidney. The nephron is the functional unit of the kidney and while the mechanism of damage depends on the underlying cause of renal disease, as nephrons become damaged and fail, remaining nephrons compensate for loss of function by hyperfiltration secondary to raised intra-glomerular pressure. This causes ‘bystander’ damage with secondary nephron loss. This vicious cycle is illustrated in Fig. 18.5. The patient remains well until so many nephrons are lost that the GFR can no longer be maintained despite activation of compensatory mechanisms. As a consequence there is a progressive decline in kidney function.
CKD arises from a variety of causes (Table 18.2), although by the time a patient has established CKD it may not be possible to identify the exact cause. However, attempting to establish the cause is useful in the identification and elimination of reversible factors, to plan for likely outcomes and treatment needs, and for appropriate counselling when a genetic basis is established. The causes of CKD listed in Table 18.2 are ordered according to prevalence. It is important to note the prevalence of these factors is different in CKD and end stage renal disease. In end stage renal disease, diseases such as adult polycystic kidney disease (APKD) are overrepresented and ischaemic/hypertensive nephropathy underrepresented. The reasons for this are that individuals with APKD are likely to survive to reach end stage renal disease while those with diabetes or ischaemic renal damage may succumb to cardiovascular disease before end stage renal disease is reached.
Table 18.2 Primary diagnosis in renal replacement therapy patients by age and gender (Renal Registry, 2008)
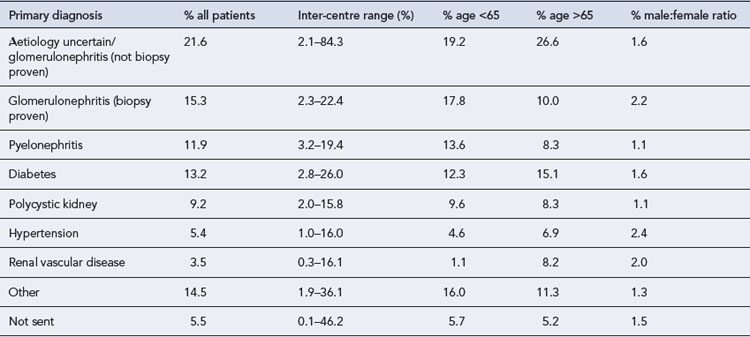
Ischaemic/hypertensive renal disease
Ischaemic nephropathy has traditionally been referred to under perfusion of the kidneys caused by renal artery stenosis. This explanation has fallen from favour recently and this term is now taken to mean impairment of renal function beyond occlusion of the main renal arteries. Hypertension results in atherosclerosis which can cause occlusive renovascular disease and small vessel damage. In patients with significant large vessel occlusive disease arteriolar nephrosclerosis, interstial fibrosis and glomerular collapse may be present (Lerman and Textor, 2001). These diagnoses account for around 30% of CKD and a smaller proportion of end stage renal disease. The effective management of hypertension is crucial to reduce renal damage.
Metabolic diseases
Diabetes mellitus is the most common metabolic disease that leads to CKD, whilst the predominant lesion is glomerular and referred to as diabetic nephropathy. Diabetes accounts for around 13% of CKD (see Table 18.2) and is associated with faster renal deterioration than other pathologies: these patients are at very significant cardiovascular risk by virtue of both CKD and diabetes. Both type 1 and 2 diabetes can result in diabetic nephrophy, patients with type 1 diabetes usually present with renal complications at a younger age and may benefit from combined kidney and pancreas transplantation. Patients with diabetes may present with no proteinuria, micro albuminuria or overt proteinuria, though as the level of proteinuria increases the GFR usually declines and in many patients this represents an inexorable decline towards end stage renal disease.
Clinical manifestations
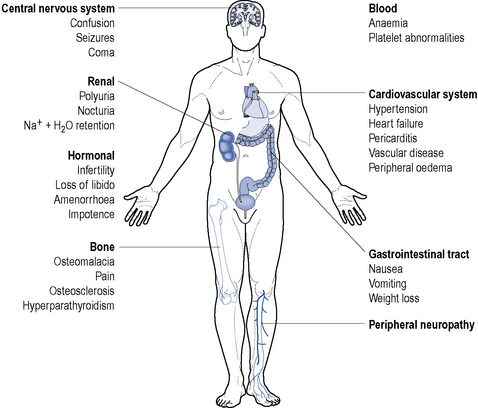
End stage renal disease is characterised by the requirement of renal replacement therapy to sustain life and it is often accompanied by uraemia, anaemia, acidosis, osteodystrophy, neuropathy and is frequently accompanied by hypertension, fluid retention and susceptibility to infection (Fig. 18.6). It results from a significant reduction in the excretory, homeostatic, metabolic and endocrine functions of the kidney that occur over a period of months or years.
In the following section, the clinical features of CKD are described, along with the pathogenesis.
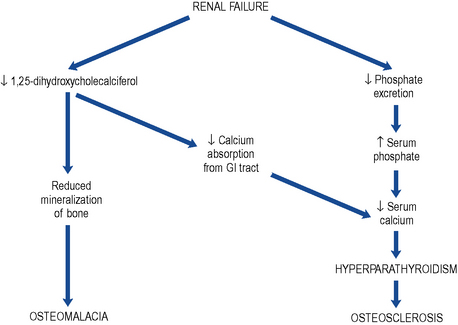
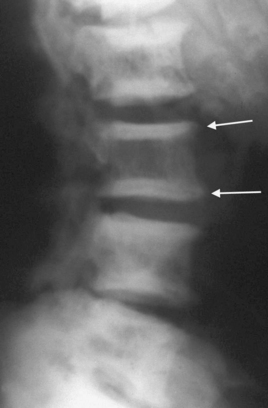
Bone disease (renal osteodystrophy)
Renal osteodystrophy describes the four types of bone disease associated with CKD:
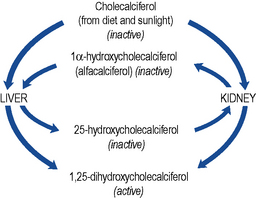
Cholecalciferol, the precursor of active vitamin D, is both absorbed from the gastro-intestinal tract and produced in the skin by the action of sunlight. Production of active vitamin D, 1,25-dihydroxycholecalciferol (calcitriol), requires the hydroxylation of the colecalciferol molecule at both the 1α and the 25 position (Fig. 18.7).
The deficiency in vitamin D with the consequent reduced calcium absorption from the gut in combination with the reduced renal tubular reabsorption results in hypocalcaemia (Fig. 18.8).
Electrolyte disturbances
Sodium
Serum sodium levels can be relatively normal even when creatinine clearance is very low. However, patients may exhibit hypo- or hypernatraemia depending upon the condition and therapy employed (see Table 18.3).
Table 18.3 Causes and mechanism of serum sodium abnormalities in chronic kidney disease
| Mechanism | Cause/effect | |
|---|---|---|
| Hypernatraemia | Sodium overload | Drugs, for example, antibiotic sodium salts |
| Hypotonic fluid loss | Osmotic diuresis | |
| Sweating | ||
| ↓ Water intake | Unconsciousness | |
| Hyponatraemia | Dilution by intracellular water movement | Mannitol |
| Hyperglycaemia | Water overload | Acute dilution by intravenous fluids, for example, 5% dextrose infusion |
| Excessive intake | ||
| Congestive cardiac failure | ||
| Nephrotic syndrome |