Chapter 5 Choosing the project
Making the decision
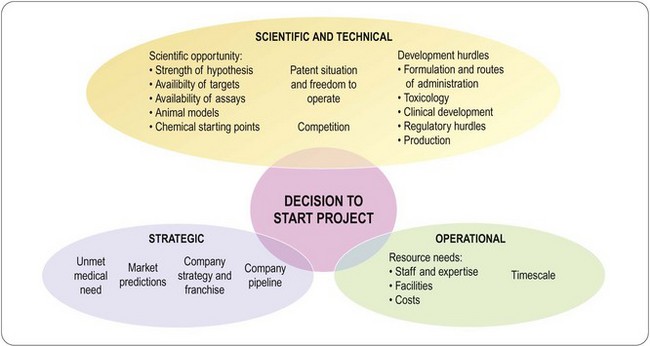
The main factors that need to be considered are summarized in Figure 5.1.
Strategic issues
Unmet medical need
• How close do we come to ‘free’? In other words, can we be more cost-effective?
• Compliance is important: a drug that is not taken cannot work. Once-daily oral dosing is convenient, but would a long-lasting injection be a better choice for some patients?
• If an oral drug exists, can it be improved, for example by changing the formulation?
• The reduction of side effects is an area where there is often a medical need. Do we have a strategy for improving the side-effect profile of existing drugs?
• Curing a condition or retarding disease progression is preferable to alleviating symptoms. Do we have a strategy for achieving this?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree