Chapter 22 Cholinergic and antimuscarinic (anticholinergic) mechanisms and drugs
Acetylcholine antagonists that block the nicotine-like effects (neuromuscular blockers and autonomic ganglion blockers) are described elsewhere (Ch. 19).
Cholinergic drugs (cholinomimetics)
• For myasthenia gravis, both to diagnose (edrophonium) and to treat symptoms (neostigmine, pyridostigmine, distigmine).
• To lower intraocular pressure in chronic simple glaucoma (pilocarpine).
• To bronchodilate patients with airflow obstruction (ipratropium, tiotropium).
• To improve cognitive function in Alzheimer’s disease (rivastigmine, donepezil).
Classification
Direct-acting (receptor agonists)
• Choline esters (bethanechol, carbachol), which act at all sites, like acetylcholine, but are resistant to degradation by acetylcholinesterases (AChE; see Fig. 22.1). Muscarinic effects are much more prominent than nicotinic (see p. 373).
• Alkaloids (pilocarpine, muscarine) act selectively on end-organs of postganglionic, cholinergic neurones. Effects are exclusively muscarinic.
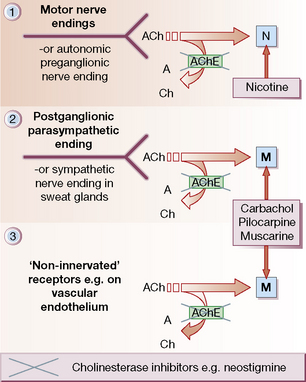
Sites of action (Fig. 22.1)
• Autonomic nervous system (Fig. 22.1, sites 1 and 2).
• Neuromuscular junction (Fig. 22.1, site 1).
• Central nervous system (CNS).
• Non-innervated sites: blood vessels, chiefly arterioles (Fig. 22.1, site 3).
Acetylcholine is released from nerve terminals to activate a post-synaptic receptor, except on blood vessels, where the action of cholinergic drugs is unrelated to cholinergic ‘vasodilator’ nerves. It is also produced in tissues unrelated to nerve endings, e.g. placenta and ciliated epithelial cells, where it acts as a local hormone (autacoid) on local receptors.
Pharmacology
Autonomic nervous system
• Eye: miosis and spasm of the ciliary muscle occur so that the eye is accommodated for near vision. Intraocular pressure falls.
• Exocrine glands: there is increased secretion most noticeably from salivary, lachrymal, bronchial and sweat glands. The last are cholinergic, but anatomically part of the sympathetic system; some sweat glands, e.g. axillary, may be adrenergic.
• Heart: bradycardia occurs with atrioventricular block, and eventually cardiac arrest.
• Bronchi: there is bronchoconstriction and mucosal hypersecretion that may be clinically serious in asthmatic subjects, in whom cholinergic drugs should be avoided if possible.
• Gut: motor activity is increased and may cause colicky pain. Exocrine secretion is also increased. Tone in sphincters falls, which may cause defaecation (anal sphincter) or acid reflux/regurgitation (oesophageal sphincter).
• Urinary bladder and ureters contract and the drugs promote micturition.
Choline esters
Acetylcholine
Acetylcholine was first injected intravenously as a therapeutic convulsant in 1939, in the reasonable expectation that the fits would be less liable to cause fractures than those following therapeutic leptazol (pentylenetetrazole) convulsions. Recovery rates of up to 80% were claimed in various psychotic conditions. Enthusiasm began to wane, however, when it was shown that the fits were due to anoxia resulting from cardiac arrest and not to pharmacological effects on the brain.1
The following description is typical:
Lachrymation, sweating and borborygmi were prominent. The deep reflexes became diminished. The patient then relaxed and ‘lay quietly in bed – cold moist and gray. In about 90 seconds, flushing of the face marked the return of the pulse’. The respiratory rate rose and consciousness returned in about 125 seconds. The patients sometimes micturated but did not defaecate. They ‘tended to lie quietly in bed after the treatment’. ‘Most of the patients were reluctant to be retreated.’2
Alkaloids with cholinergic effects
(see also p. 151) is a social drug that lends its medicinal use as an adjunct to stopping its own abuse as tobacco. It is available as gum to chew, dermal patches, a nasal spray or an inhalator. These deliver a lower dose of nicotine than cigarettes and appear to be safe in patients with ischaemic heart disease. The patches are slightly better tolerated than the gum, which releases nicotine in a more variable fashion depending on the rate at which it is chewed and the salivary pH, which is influenced by drinking coffee and carbonated drinks. Nicotine treatment is reported to be nearly twice as effective as placebo in achieving sustained withdrawal from smoking (18% versus 11% in one review).3 Treatment is much more likely to be successful if it is used as an aid to, not a substitute for, continued counselling. Bupropion is possibly more effective than the nicotine patch4 (see also p. 152) and the partial nicotinic agonist, varenicline, slightly more effective still. The efficacy of varenicline is tempered by its ability to cause suicidal ideation and behaviour.
from a South American plant (Pilocarpus spp.), acts directly on muscarinic receptors (see Fig. 22.1); it also stimulates and then depresses the CNS. The chief clinical use of pilocarpine is to lower intraocular pressure in primary open-angle glaucoma (also called chronic simple or wide-angle glaucoma), as an adjunct to a topical β-blocker; it produces miosis, opens drainage channels in the trabecular network and improves the outflow of aqueous humour. Oral pilocarpine is available for the treatment of xerostomia (dry mouth) in Sjögren’s syndrome, or following irradiation of head and neck tumours. The commonest adverse effect is sweating, an effect actually exploited in a diagnostic test for cystic fibrosis.
Poisoning with these fungi may present with antimuscarinic, cholinergic or GABAergic effects. All have CNS actions. Happily, poisoning by Amanita muscaria is seldom serious, but species of Inocybe contain substantially larger amounts of muscarine (see Ch. 10). The lengths to which humans are prepared to go in taking ‘chemical vacations’ when life is hard are shown by the inhabitants of eastern Siberia, who used Amanita muscaria recreationally for its cerebral stimulant effects. They were apparently prepared to put up with the autonomic actions to escape briefly from reality – so much so that when the fungus was scarce in winter they were even prepared to drink their own urine to prolong the experience. Sometimes, in generous mood, they would even offer their urine to others as a treat.
Anticholinesterases
At cholinergic nerve endings and in erythrocytes there is a specific enzyme that destroys acetylcholine, true cholinesterase or acetylcholinesterase. In various tissues, especially plasma, there are other esterases that are not specific for acetylcholine but that also destroy other esters, e.g. suxamethonium, procaine (and cocaine) and bambuterol (a prodrug that is hydrolysed to terbutaline). Hence, they are called pseudocholinesterases. Chemicals that inactivate these esterases (anticholinesterases) are used in medicine and in agriculture as pesticides. They act by allowing naturally synthesised acetylcholine to accumulate instead of being destroyed. Their effects are explained by this accumulation in the CNS, neuromuscular junction, autonomic ganglia, postganglionic cholinergic nerve endings (which are principally in the parasympathetic nervous system) and in the walls of blood vessels, where acetylcholine has a paracrine5 role not necessarily associated with nerve endings. Some of these effects oppose one another, e.g. the effect of anticholinesterase on the heart will be the result of stimulation at sympathetic ganglia and the opposing effect of stimulation at parasympathetic (vagal) ganglia and at postganglionic nerve endings.
is an alkaloid, obtained from the seeds of the West African Calabar bean (spp. Physostigma), which has had long use both as a weapon and as an ordeal poison.6 It acts for a few hours. It has been shown to have some efficacy in improving cognitive function in Alzheimer-type dementia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree