Cholecystostomy, Cholecystectomy, and Intraoperative Evaluation of the Biliary Tree
O. James Garden
Introduction
Despite laparoscopic cholecystectomy having become firmly established in the surgical treatment of gallstone disease in the last 20 years, there remains a requirement for the surgeon to understand the fundamentals of the open approach. Open cholecystectomy may be necessary as an incidental undemanding procedure during more complex surgery of the liver, bile duct, or pancreas. However, a more challenging procedure may result in patients undergoing conversion following failed laparoscopic dissection due to difficult anatomy or more severe gallbladder disease. The modern surgeon may have acquired greater expertise in the minimally invasive procedure and have little experience of open cholecystectomy. However, there is a need to apply the same safe principles in circumstances that may be more challenging.
Calot’s (hepatocystic) triangle is bounded by the gallbladder, the right side of the common hepatic duct, and the liver. Elevation of the base of the gallbladder from the lower portion of the cystic plate and clearance of this triangle allows clear identification of the cystic structures during cholecystectomy. The cystic duct is approximately 2 cm in length and 3 mm in diameter, joining the common hepatic duct at an acute angle to form the common bile duct (CBD). Given that this junction may occur at any level down to the ampulla, the length of the cystic duct is variable. The cystic artery normally rises from the right hepatic artery in the hepatocystic triangle and runs for 1 to 2 cm to the upper border of the gallbladder, superior to the insertion of the cystic duct. Anterior and posterior branches may be evident at the point of contact with the gallbladder. Where the cystic artery arises from an aberrant right hepatic artery via the superior mesenteric vessel, the cystic artery, and not the cystic duct, usually lies in the free edge of the hepatoduodenal ligament.
The gallbladder lies on a fibrous area referred to as the cystic plate, which is part of the perihilar system of fibrous tissue. The cystic plate attaches directly onto the anterior surface of the right portal pedicle. The hepatic parenchyma lies deep to the cystic plate, through which small bile ducts may penetrate to enter the gallbladder. These ducts of Luschka consist of accessory ducts less than 1 mm in diameter. In approximately 10% of patients, there is a large peripheral bile duct immediately deep to the plate, dissection of which may cause bile leakage. The origin of the middle hepatic vein also lies at a variable depth beneath the cystic plate and may be entered inadvertently.
Between the gallbladder muscularis and the cystic plate, a thin layer of areolar tissue thickens progressively from the top of the gallbladder downward. The posterior surface of the cystic artery and bile duct will be reached when the areolar tissue is left on the cystic plate during dissection of the gallbladder from the liver. Should dissection be undertaken deep into the cystic plate, the right portal pedicle may be breached and may result in injury to its structures including the right hepatic duct and artery.
The common hepatic duct is formed by the union of the left and right ducts and runs from the base of segment IV in the right edge of the hepatoduodenal ligament for 2 to 3 cm before joining the cystic duct to join the CBD. The CBD courses above the duodenum for 3 to 4 cm before it passes behind the first part of the duodenum, within or behind the pancreas to enter the second part of the duodenum. The CBD external diameter varies from 3 to 13 mm when distended to physiologic pressures. Low insertions of the right hepatic ducts are the most common anomaly and in 2% of patients, the right hepatic sectional (sectoral) ducts join the common hepatic duct close to the cystic duct entry.
The right hepatic artery passes behind the bile duct in 80% of cases and, where there is severe inflammation, fibrosis can cause the artery to become adherent to the gallbladder to form an inverse U-loop. This tethering of the anatomy may place the right hepatic artery at risk if it is mistaken for the cystic artery during cholecystectomy. The CBD derives its arterial blood supply from a plexus of vessels arising from two marginal arteries running at the 3 and 9 o’clock positions. These vessels derive their principal blood supply inferiorly.
From autopsy studies, it has been established that 12% of men and 24% of women of all ages have gallstones present and 10% to 30% of gallstones become symptomatic. Patients may present with an acute episode of pain from biliary colic, an ill-defined collection of symptoms often referred to as flatulent dyspepsia, chronic recurrent abdominal discomfort from repeated episodes of biliary colic, or acute cholecystitis. Pain may commence in the epigastrium but will localize to the right upper quadrant if there is local inflammation of the gallbladder. There may be systemic illness if the gallbladder contents become infected. Without intervention, toxemia may result if the gallbladder becomes the seat of an empyema or possibly develops gangrene and perforation. An empyema would normally produce pain, right upper quadrant tenderness, and swinging pyrexia, although in the elderly patient these symptoms may not always be obvious until the patient presents with an acute abdomen and collapse. CBD stones may present with features of obstructive jaundice with passage of pale stool and dark urine. Accompanying biliary sepsis represents a significant risk to the patient and presents with pain, fever, and frequently rigors. The development of associated medical signs such as atrial fibrillation in the elderly patient may signal the need for urgent intervention. Jaundice may occasionally result from external compression of the CBD from an inflamed and edematous gallbladder (Mirizzi’s syndrome).
The diagnosis of gallstone disease is often suspected on clinical grounds but is confirmed by appropriate laboratory and radiological investigation. Liver function tests should be performed routinely since they may be abnormal in the presence of CBD stones. Although not always specific for choledocholithiasis, serum levels of bilirubin, alkaline phosphatase, and gamma-glutamyl transpeptidase are the most sensitive tests used routinely. An elevated white blood cell count may indicate sepsis. Ultrasonography (US) is most widely used to
confirm the diagnosis of cholelithiasis. Other imaging modalities are used rarely although cholecystokinin-stimulated hydroxy iminodiacetic acid scanning may be useful in the diagnosis of atypical biliary pain such as in biliary dyskinesia. US may detect evidence of chronic disease and the presence of a thickened wall and contracted gallbladder may signify that open cholecystectomy may be more likely to succeed than a laparoscopic approach. Edema may be evident in acute cholecystitis and dilation of the CBD may signify the presence of ductal calculi. These are more likely to be detected by magnetic resonance cholangiography, which may be used to plan the approach to managing choledocholithiasis. Endoscopic retrograde cholangiography allows detection and removal of ductal calculi following sphincterotomy and may be required to decompress the biliary tree preoperatively in Mirizzi’s syndrome.
confirm the diagnosis of cholelithiasis. Other imaging modalities are used rarely although cholecystokinin-stimulated hydroxy iminodiacetic acid scanning may be useful in the diagnosis of atypical biliary pain such as in biliary dyskinesia. US may detect evidence of chronic disease and the presence of a thickened wall and contracted gallbladder may signify that open cholecystectomy may be more likely to succeed than a laparoscopic approach. Edema may be evident in acute cholecystitis and dilation of the CBD may signify the presence of ductal calculi. These are more likely to be detected by magnetic resonance cholangiography, which may be used to plan the approach to managing choledocholithiasis. Endoscopic retrograde cholangiography allows detection and removal of ductal calculi following sphincterotomy and may be required to decompress the biliary tree preoperatively in Mirizzi’s syndrome.
Symptomatic gallbladder disease would normally be managed definitively by cholecystectomy in the fit patient. For the patient presenting acutely, appropriate analgesia is required and resuscitation with intravenous fluids and antibiotics may be required if there is evidence of sepsis. In general, endoscopic retrograde cholangiography with sphincterotomy, removal of stones, and stent placement would be indicated in the patient with cholangitis since morbidity and mortality are lower than with urgent operative decompression of the bile duct.
For procedures that may involve associated or coincidental cholecystectomy, investigation will be driven by the underlying disease process. For example, computerized tomography or positron emission tomography may be required to image liver tumor and stage the patient before cholecystectomy and liver resection is performed whereas magnetic resonance cholangiopancreatography may be required to evaluate pathology such as a choledochal cyst prior to its removal with the gallbladder.
Gallstones may cause multiple biliary tract problems, requiring surgery; the most common indication for cholecystectomy is biliary colic caused by chronic cholecystitis and cholelithiasis. Acute cholecystitis, secondary to obstruction of the cystic duct by a gallstone, is the second most common reason for removal of the gallbladder. Empyema and perforation are sinister complications of gallstone disease that may necessitate open surgery. Pancreatitis and cholangitis caused by obstruction of the pancreatic duct and/or distal CBD by a stone are generally the third most common indication for cholecystectomy. Incidental open cholecystectomy may be required during liver resection, pancreaticoduodenectomy, open surgery for associated benign pancreatic pathology, such as a pseudocyst or chronic pancreatitis, or for primary biliary pathology, such as choledochal cyst.
Despite the high incidence of gallstone disease in many countries, approximately 75% of patients with gallstones are asymptomatic. Although there are conflicting data, it would appear that less than 20% of patients with “silent gallstones” develop complications within a 10-year period. In the United Kingdom more than 40,000 cholecystectomies are performed each year, whereas in the United States 500,000 are performed annually. The need for conversion from laparoscopic to open cholecystectomy may be anticipated in patients over the age of 65 years, in male patients, those experiencing multiple previous attacks of biliary colic or during acute admission with acute cholecystitis. Primary open cholecystectomy may be considered in as many as 5% of patients requiring removal of the gallbladder and would include patients such as those undergoing laparotomy for peritonitis or other intra-abdominal pathology when gallbladder disease has not been suspected preoperatively. Other possible indications for open cholecystectomy are listed (Table 1) although it is accepted that the threshold for an open rather than a laparoscopic approach will vary with the experience and expertise of the surgeon.
Cholecystostomy
With improvements in anesthesia and surgery, cholecystostomy is undertaken infrequently and is performed generally by radiologic techniques under local, regional, or general anesthesia. It is a procedure that should be considered when cholecystectomy is deemed unsafe in patients at high risk related to multisystem organ failure; severe pulmonary, renal, or cardiac disease; and recent myocardial infarction. Safe cholecystectomy may be precluded by a local condition such as cirrhosis with portal hypertension; acalculous cholecystitis after severe trauma, burns, or surgery; and where there is empyema or gangrene of the gallbladder.
Open or percutaneous cholecystostomy under local anesthesia should not be considered a minor procedure in the sick patient, and a skilled anesthetist should be present since the patient should be well sedated and oxygenated. Preoperative or operative localization of the gallbladder using US is invaluable in selecting the most appropriate incision site. Ample local anesthetic with 0.5% lidocaine is used to infiltrate the skin muscle and peritoneum. A transverse muscle-splitting incision is preferred for the open procedure and, if the incision site has been well chosen, the gallbladder will be seen immediately on entering the peritoneum.
A purse-string 3-0 polydioxanone suture is placed in the fundus of the gallbladder. Having placed packs or swabs around the isolated gallbladder, a trocar is used to decompress the gallbladder. A disposable 5-mm laparoscopic port is ideal as the sharp trocar avoids unnecessary trauma to the operating field. The contents of the gallbladder are aspirated and a sample of bile is obtained for culture. In the absence of empyema or gangrene, an attempt should be made to remove the stones with forceps or a scoop. A stone impacted in the neck of the gallbladder or cystic duct can be dislodged usually by external digital manipulation, stone forceps, or a balloon catheter. Removal of the obstructing stone is important to prevent a subsequent mucous fistula.
On removal of the stones, a No. 20 to 24 French gauge Foley or similar catheter is placed through a separate stab incision in the abdominal wall and is secured within the fundus of the gallbladder, using the purse-string suture (Fig. 1). Injudicious attempts at securing the gallbladder to the abdominal wall should be avoided, although this may be achieved frequently by inflating the catheter balloon, which allows the fundus of the gallbladder to be opposed against the parietal peritoneum. The incision is closed using 0 polydioxanone to peritoneum and sheath. If there has been considerable contamination of the wound, it may be elected not to close the skin edges.
Table 1 Preoperative Indications for Primary Open Cholecystectomy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Given the circumstances under which cholecystostomy is performed, it is not surprising that the procedure carries a higher
morbidity and mortality than cholecystectomy. In those patients who recover from the procedure, the cholecystostomy tube can be removed at 6 to 8 weeks if patency of the biliary tract can be demonstrated. For those patients with residual stones in the gallbladder, these may be removed through the tract by percutaneous maneuvers using image intensification or 5-mm flexible choledochoscope. Dissolution techniques are not well tolerated and, in those patients who make a satisfactory recovery, cholecystectomy should be considered. In the elderly, infirm patient or those with comorbid disease, it may be wise to defer cholecystectomy indefinitely.
morbidity and mortality than cholecystectomy. In those patients who recover from the procedure, the cholecystostomy tube can be removed at 6 to 8 weeks if patency of the biliary tract can be demonstrated. For those patients with residual stones in the gallbladder, these may be removed through the tract by percutaneous maneuvers using image intensification or 5-mm flexible choledochoscope. Dissolution techniques are not well tolerated and, in those patients who make a satisfactory recovery, cholecystectomy should be considered. In the elderly, infirm patient or those with comorbid disease, it may be wise to defer cholecystectomy indefinitely.
Open Cholecystectomy
Preoperative preparation would involve appropriate thromboembolic prophylaxis according to individual patient risk and administration of a suitable prophylactic antibiotic that would cover common biliary commensal organisms such as cefuroxime or amoxicillin and clavulanic acid. The first dose should be administered before the procedure and preferably within 30 minutes before skin incision. In those patients who have undergone conversion from laparoscopic cholecystectomy, the antibiotic should be administered as soon as is practicable.
Of the frequently employed incisions for cholecystectomy, a right-sided subcostal (Kocher) or transverse incision provides the optimum exposure. The incision should normally be of sufficient size to provide adequate exposure and to admit the examining hand. In the era of laparoscopic cholecystectomy, there appears little rationale for the so-called minicholecystectomy approach in the patient for whom adequate access will be required to deal with a difficult situation.
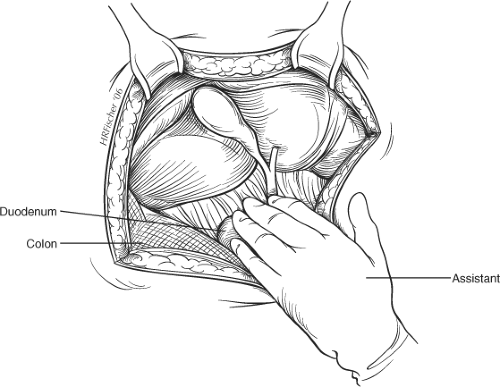
The abdomen is explored to exclude other intra-abdominal pathology and a hand is passed over the right lobe of the liver to allow air to enter the subphrenic space. The costal margin is retracted and the use of a fixed mechanical retractor is helpful in providing exposure and in freeing the assistant’s hands during the operative procedure. Placement of a pack or small swab above the liver may increase exposure of the subhepatic region in some patients. Moist gauze swabs are placed to the right and inferiorly to displace the hepatic flexure of the colon and to the left to displace the stomach away from the operating field. A further pack is used under the assistant’s hand to allow traction caudally (Fig. 2).
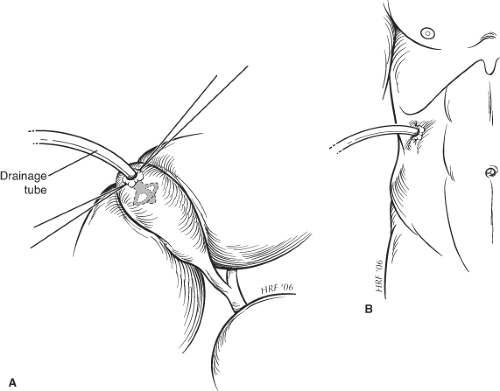 Fig. 1. Method for securing drainage tube during cholecystostomy. The drainage tube is secured to the gallbladder wall by a purse string suture (A) and to the abdominal wall by a skin suture (B). |
Adhesions between the gallbladder and gastrocolic omentum or transverse colon may be divided using bipolar diathermy scissors. Placement of a small, moist swab into the subhepatic space to the right and behind the gallbladder will prevent inadvertent spillage of infected bile or stones, which may not otherwise be retrieved easily on completion of the operation. Preliminary decompression of the gallbladder avoids uncontrolled spillage of infected bile during dissection if the gallbladder is tense and acutely inflamed. A trocar can be inserted through the center of a 3-0 polydioxanone purse-string suture placed at the fundus of the gallbladder in an easily accessible location. The gallbladder contents are
aspirated before withdrawal of the trocar and closure of the resulting defect by tying the purse-string suture.
aspirated before withdrawal of the trocar and closure of the resulting defect by tying the purse-string suture.
With the assistant providing exposure and traction caudally and to the left, a Kelly clamp is placed on the fundus of the gallbladder, which is retracted out of the wound and displaced caudally. A second Kelly clamp is applied further down the gallbladder on Hartmann’s pouch but away from the cystic duct (Fig. 3). The peritoneum overlying Calot’s triangle is incised and the dissection is continued close to the gallbladder, displacing the cystic node and the fatty, areolar tissues to expose the cystic duct and artery. As for laparoscopic cholecystectomy, the incision to the peritoneum can be continued posteriorly as the Kelly clamp on the infundibulum is displaced upward and medially. This allows clear identification of the cystic duct and artery using a right-angled Lahey or Kittner dissector. The cystic artery is identified and traced onto the gallbladder far enough to ensure that it is not the right hepatic artery. Two braided 2-0 ties are passed around the artery, which is divided between these (Fig. 3). The cystic duct is identified by blunt dissection with the right-angled clamp and circled with a ligature, which can be double-looped around the duct to provide temporary atraumatic occlusion of the duct. This maneuver helps prevent small stones or debris being forced down into the CBD during dissection and facilitates performance of a cholangiogram through the cystic duct (see below). If a cholangiogram is not undertaken, the duct can be divided between two ties.
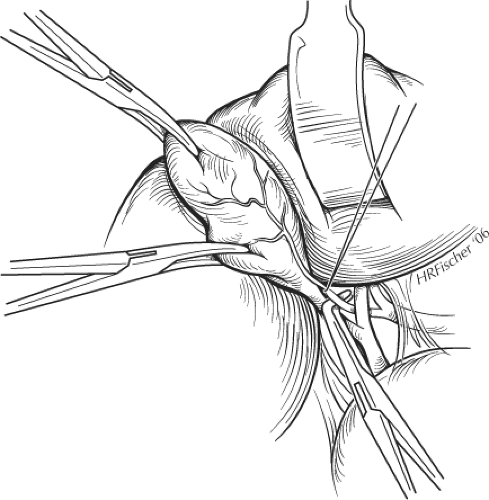 Fig. 3. Placement of clamps on the gallbladder and dissection of cystic artery during cholecystectomy. |
It is inappropriate to dissect the cystic duct to its confluence with the common hepatic duct but it is important to establish that the cystic duct is free of stones at the end of cholecystectomy. It does not need to be ligated flush with the bile duct because there is no evidence that ligation of the cystic duct at its union with the gallbladder results in residual symptoms. The cystic duct can be short, and dissection may be difficult in the presence of a large, impacted stone, and the presence of acute or chronic inflammation may result in adhesions between the gallbladder and the common hepatic or bile duct, thereby concealing the cystic duct and contributing to the possibility of biliary injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree