Cholecystojejunostomy and Choledocho/Hepaticojejunostomy
Herbert R. Freund
Cholecystojejunostomy
Favorable data have been published in recent years concerning curative surgery of periampullary carcinoma. The operative mortality of pancreatoduodenectomy ranges below 3% to 5% and morbidity has dropped to 25% to 30%. Even in nonmajor referral centers, operative mortality (7% to 10%) and morbidity (30% to 40%) dropped significantly. Five-year survival rates for pancreatic cancer are reported to approach 12% to 24% for patients undergoing curative resection compared with a median survival of 6 to 10 months for patients undergoing bypass surgery or stenting by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) procedures. In various large reports, however, only 20% of patients have resectable pancreatic tumors, the remaining requiring some sort of palliation for relief of jaundice, actual or pending duodenal obstruction, or severe, often debilitating pain.
Decompression of the biliary tract obstructed by malignant periampullary lesions is indicated for the relief of obstructive jaundice and its ensuing severe and often intolerable pruritus. There are also ample data to suggest that stenting in malignant obstructive jaundice improves physical and functional well-being. Three viable options are available for the palliation of obstructive
jaundice caused by periampullary malignant lesions in patients whose disease is determined to be unresectable:
jaundice caused by periampullary malignant lesions in patients whose disease is determined to be unresectable:
Percutaneous transhepatic external or internal permanent catheter drainage of the biliary system.
Endoscopic papillotomy with insertion of an internal biliary–duodenal stent catheter.
Operative biliary–enteric bypass by cholecystojejunostomy or choledochojejunostomy/hepaticojejunostomy with or without a gastroenteric bypass.
Without getting into the yet unresolved argument about whether radiologic or endoscopic stents are superior to a biliary–enteric surgical bypass, we believe that a surgical biliary–enteric bypass has a viable role in the palliation of patients who have periampullary carcinomas, particularly in younger more fit patients, who are likely to survive a reasonable length of time. These patients are also the ones in whom an additional gastrojejunostomy is advocated for actual or pending mechanical gastric outlet obstruction, because comparable postoperative morbidity and mortality have been reported after “double bypass,” or biliary diversion only. As a rule we prefer to perform a “double bypass,” namely, a cholecystojejunostomy, choledochojejunostomy, or hepaticojejunostomy and a gastrojejunostomy.
This approach receives further support from a recent meta-analysis of three prospective studies (218 patients) comparing prophylactic palliative gastroenterostomy and bilio-digestive anastomosis with no bypass or a bilio-digestive anastomosis alone that has shown that for patients with gastroenterostomy the chance for gastric outlet obstruction was significantly lower with no added operative morbidity or mortality.
The use of the gallbladder for internal biliary drainage is quick, simple, effective, and safe, and is the biliary conduit of choice when the cystic duct is patent and enters the common duct well away (1 cm) from the tumor mass. The gallbladder should not be used if it was previously diseased (chronic cholecystitis or cholecystolithiasis, or both), or when the cystic duct is narrow or enters the common duct close to the tumor. In such situations, the common hepatic duct is the preferred conduit for bypass, and the gallbladder is removed. When creating a cholecystojejunostomy, which is the simplest and quickest bypass, a loop of jejunum is the preferred enteric component of the bypass, whereas with a choledochojejunostomy/hepaticojejunostomy, the Roux-en-Y technique is applied.
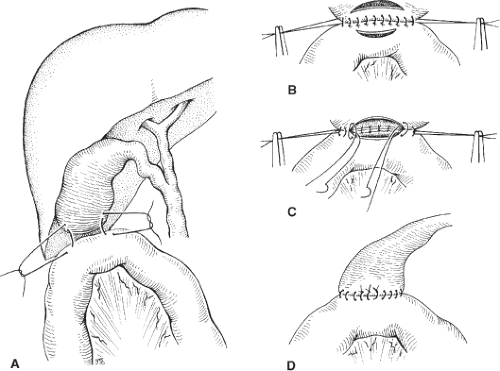
Cholecystojejunostomy with a simple loop bypass is almost exclusively used in patients who were operated for cure and were found during exploration to have locally advanced disease, possibly small liver metastases or peritoneal implants. The first loop of jejunum that easily and comfortably reaches the subhepatic space is passed in an antecolic manner without tension and approximated to the gallbladder with a posterior row of seromuscular interrupted absorbable (4-0 or 3-0) sutures (Fig. 1A,B). On the jejunum, the sutures are placed parallel and adjacent to the edge of the mesenterium. Using electrocautery, an incision as long as possible is made in the body of the gallbladder, and the bile evacuated and sent for culture. A similar, but shorter, incision is made on the antimesenteric part of the approximated jejunum (Fig. 1B). Beginning in the middle and suturing toward the corners, the full thickness of the gallbladder and of the jejunum are anastomosed with 3-0 or 4-0 monofilament absorbable interrupted sutures. This creates the posterior wall of the anastomosis with all sutures tied on the inside (Fig. 1C). Turning both corners, sutures are placed in an inside–outside–outside–inside fashion from both sides of the anterior wall with ties on the inside (Fig. 1C). The last sutures on the anterior wall (simple or gumby sutures) are all placed before tying them with their knots on the outside (Fig. 1D). We rarely approximate the anterior serosal layer, although, if the gallbladder is large and redundant enough, it could be done. In our experience, leakage from a cholecystojejunostomy is extremely rare, but we still leave a closed suction drainage for 2 to 4 days. It is our practice to always add a small, sutured, or GIA-stapled gastrojejunostomy distal to the cholecystojejunostomy.
Technical and technological advances in laparoscopic surgery make it possible to safely perform the double bypass procedure laparoscopically, which results in reduced operative injury and possibly in a shorter and easier postoperative course and more rapid convalescence.
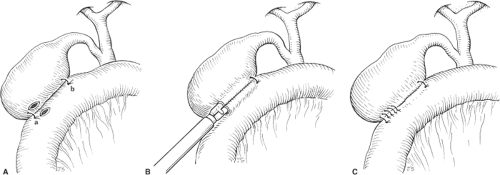
Laparoscopic cholecystojejunostomy is accomplished through three to five trocars. The first jejunal loop to comfortably reach the gallbladder is attached with one or two anchoring sutures to the gallbladder (Fig. 2A). A small incision is made close to the proximal anchoring suture (Fig. 2A) in the gallbladder and the adjacent jejunum. The jaws of the endoscopic linear stapler 3.5/4.5 stapler or its equivalent are introduced through these two incisions and pushed to its full 3-cm length, closed, and fired (Fig. 2B).
The two entry sites, now combined into one, can be closed by a second stapler firing or with interrupted sutures (Fig. 2C).
The two entry sites, now combined into one, can be closed by a second stapler firing or with interrupted sutures (Fig. 2C).
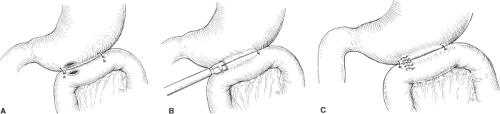
Laparoscopic gastrojejunostomy is technically a similar straightforward procedure that provides excellent palliation for the 10% to 20% of patients who have unresectable pancreatic cancer and subsequently develop duodenal obstruction. Here too, two anchoring sutures are placed to approximate the jejunum to the stomach (Fig. 3A). Two small incisions in the stomach and the adjacent jejunum enable the introduction and firing of a 3.5 or 4.5 endoscopic linear stapler (Fig. 3B). The common opening left after removal of the stapler is stapled or sutured (Fig. 3C).
We believe that the use of the laparoscopic technique for the palliation of malignant biliary obstruction is associated with a lower operative risk while offering longer palliation without the risk of gastric outlet obstruction, recurrent cholangitis, and repeated endoscopic stent placement and change.
Choledochojejunostomy/Hepaticojejunostomy
The main indications for a choledochojejunostomy/hepaticojejunostomy are benign, mainly iatrogenic, biliary strictures and malignant obstruction of the biliary system caused by peri-ampullary tumors. Rare indications for choledochojejunostomy/hepaticojejunostomy are direct trauma to the biliary system, choledochal cyst reconstruction, and select instances of sclerosing cholangitis in which areas of dilatation occur. Because biliary tract strictures are essentially iatrogenic in 97% of instances (0.5% incidence of duct injury in laparoscopic cholecystectomy), and each unsuccessful repair poses increased morbidity and mortality, reconstruction should restore long-lasting primary functional and anatomic integrity at the first attempt of repair.
The diagnosis should be established, and the anatomy of the biliary duct system and the nature and location of the obstruction should be characterized by PTC, endoscopic retrograde cholangiography, or both. At the same time, jaundiced patients can undergo drainage by either a percutaneous catheter or an endoprosthesis. The presence of such catheters, although reported to carry an increased operative morbidity, is a
tremendous help for the surgeon exploring a scarred previously damaged or transected ductal system, particularly if this attempt to repair is not the first.
tremendous help for the surgeon exploring a scarred previously damaged or transected ductal system, particularly if this attempt to repair is not the first.
The transhepatic catheter might also be left in place during an operation for stenting and future imaging and removed only after the safety and patency of the anastomosis is ascertained by injection of contrast material 1 to 2 weeks following repair.
Most patients who require a choledochojejunostomy/hepaticojejunostomy may have experienced cholangitis and jaundice, and should therefore receive antibiotics, parenteral vitamin K, and possibly fresh frozen plasma perioperatively. Nutritional support should be offered when and where indicated. In our experience, most patients who have a diseased biliary system also harbor infected bile, with the most common pathogens being Escherichia coli, Klebsiella species, and various streptococci. However, because many of these patients have had previous biliary tract operations and several interventions and instrumentations, infections with hospital-acquired anaerobes or resistant bacteria should be considered in the choice of perioperative antibiotics. Our preference is the combination of gentamicin and ampicillin, amikacin and ampicillin, or a third-generation cephalosporin. Because the colon can be densely adherent to the gallbladder bed and liver, requiring meticulous dissection and separation, large bowel preparation is advised, but certainly not absolutely indicated.
The biliary tract can be approached through a long right subcostal or a right paramedian incision. However, the best possible exposure is offered by the bilateral subcostal, better known as the “bucket-handle incision,” with or without the midline xiphoid extension. Figure 4 depicts the anatomic situation following the mandatory steps to release adhesions, dissection of the area of the hepatoduodenal ligament, and kocherization of the duodenum. It is important at this stage to perform meticulous dissection of the area between the undersurface of the right lobe of the liver and the duodenum. Sometimes, aspiration with a 25-gauge needle attached to a small syringe can be helpful in locating the dilated common duct. Favorable results have been reported with intraoperative sonographic detection of dilated common ducts, ductal stones, and pancreatic and ductal tumors. In dissecting out the common bile duct, the hepatic artery lying to its left is an important anatomic landmark (Fig. 4). However, one should be aware that this “textbook” arrangement of the common hepatic artery originating from the celiac trunk, giving off the left and right hepatic arteries, is found in only 55% of patients. In the other 45% of patients, variations in the hepatic arterial supply exist. Of particular importance in this respect is the right hepatic artery arising from the superior mesenteric artery, which usually passes to the liver along the right side of the portal vein and the common bile and common hepatic ducts. Also frequent are anatomic variations in the extrahepatic biliary tree, most of them related to the confluence of the right and left hepatic ducts and of the cystic duct insertion into the common hepatic duct.
Once the common duct has been identified and dissected out, two 4-0 traction sutures are placed through the dilated common-duct wall just above the area of obstruction. The common duct is ligated below the stricture with two 00 ligatures and is divided below the traction sutures (Fig. 5). The proximal end of the common duct is trimmed to achieve maximal diameter, good blood supply, and viable, free of scar duct tissue for anastomosis. Because the common hepatic and upper bile ducts depend on two axial arteries located along the lateral and medial borders of the ducts, undue mobilization and dissection should be avoided to ensure good blood supply to the anastomotic site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree