INTRODUCTION
Chlamydiae that infect humans are divided into three species, Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci, on the basis of antigenic composition, intracellular inclusions, sulfadiazine susceptibility, and disease production. The separation of the genus Chlamydia into the genera Chlamydia and Chlamydophila was controversial; in this chapter, the three chlamydiae that are pathogens of humans are considered to be in the genus Chlamydia in keeping with publications that do not support the new taxonomy. Other chlamydiae infect animals but rarely if ever infect humans. All chlamydiae exhibit similar morphologic features, share a common group antigen, and multiply in the cytoplasm of their host cells by a distinctive developmental cycle. The chlamydiae can be viewed as gram-negative bacteria that lack mechanisms for the production of metabolic energy and cannot synthesize adenosine triphosphate (ATP). This restricts them to an intracellular existence, where the host cell furnishes energy-rich intermediates. Thus, chlamydiae are obligate intracellular pathogens.
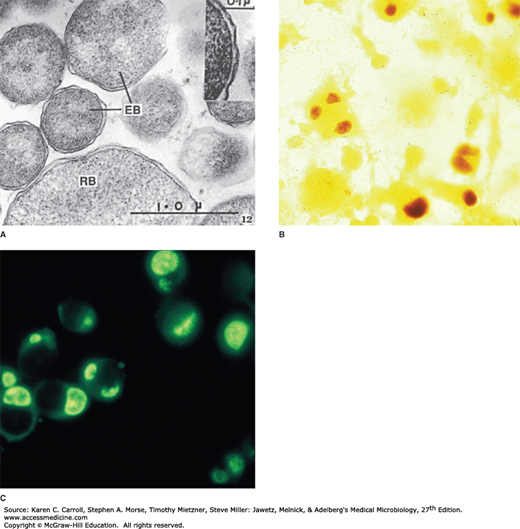
All chlamydiae share a common and unique biphasic developmental cycle. The environmentally stable infectious particle (transmissible form) is a small cell called the elementary body (EB). These are about 0.3 μm in diameter (Figure 27-1) with an electron-dense nucleoid. The EB membrane proteins have highly cross-linked membrane proteins. The EBs have a high affinity for host epithelial cells and rapidly enter them. The first step in entry involves interaction between outer membrane proteins of the EB and heparin sulfate proteoglycan of the host cells. The second step involves additional and irreversible binding to a variety of other host cell receptors. There appear to be multiple adhesins, such as OmcB, the major outer membrane protein (MOMP), glycosylated MOMP, and other surface proteins. Following adherence, the mechanisms thought to mediate entry into the host cell also vary and involve cytoskeletal rearrangements and activation of type III secretion systems and other effectors. EBs are usually seen attached near the base of microvilli, where they are subsequently engulfed by the host cell. More than one mechanism appears to be functional: receptor-mediated endocytosis into clathrin-coated pits and pinocytosis via noncoated pits. Lysosomal fusion is inhibited, creating a protected membrane-bound environment around the chlamydiae. Shortly after entry into the host cell, the disulfide bonds of the EB membrane proteins are reduced (no longer cross-linked), and the EB is reorganized into a larger structure called a reticulate body (RB) [replicative form] measuring about 0.5–1 μm (see Figure 27-1) and devoid of an electron-dense nucleoid. Within the membrane-bound vacuole, the RB grows in size and divides repeatedly by binary fission. Eventually, the entire vacuole becomes filled with EBs derived from the RBs to form a cytoplasmic inclusion. The newly formed EBs may be liberated from the host cell to infect new cells. The developmental cycle takes 48–72 hours.
FIGURE 27-1
Chlamydiae. A: Thin section electron micrograph of chlamydiae in various stages of development. EB, elementary body particles with cell walls (inset); RB, reticulate body. B: Chlamydia trachomatis grown in McCoy cells and stained with iodine. The McCoy cells stain a faint yellow in the background. The glycogen-rich intracytoplasmic inclusions of C trachomatis stain a dark brown. C: Similar growth of C trachomatis in McCoy cells stained with a fluorescein-labeled antibody against a C trachomatis species antigen. The intracytoplasmic inclusions of C trachomatis stain bright yellow-green. Faint outlines of the McCoy cells are visible. (Courtesy of J Schachter.)
In chlamydiae, the outer cell wall resembles the cell wall of gram-negative bacteria. It has a relatively high lipid content including lipopolysaccharide of low endotoxic activity. It is rigid but does not contain a typical bacterial peptidoglycan. As mentioned above, another important structural component is the MOMP encoded by ompA. MOMP antigenic variants of C trachomatis are associated with different clinical syndromes. Penicillin-binding proteins occur in chlamydiae, and chlamydial cell wall formation is inhibited by penicillins and other drugs that inhibit transpeptidation of bacterial peptidoglycan. Lysozyme has no effect on chlamydial cell walls. N-acetylmuramic acid appears to be absent from chlamydial cell walls. Both DNA and RNA are present in EBs and RBs. The RBs contain about four times as much RNA as DNA, whereas the EBs contain about equal amounts of RNA and DNA. In EBs, most DNA is concentrated in the electron-dense central nucleoid. Most RNA exists in ribosomes. The circular genome of chlamydiae is 1.04 megabases in length, encodes 900 genes, and is one of the smallest bacterial genomes.
Multiple chlamydial genomes have been sequenced, providing insight into the basic biology of the organisms. For example, chlamydiae have a type III secretion system, which may allow them to inject effector proteins into host cells as part of the infectious process (see discussion above under Developmental Cycle).
Chlamydiae have distinctive staining properties (similar to those of rickettsiae). Elementary bodies stain purple with Giemsa stain—in contrast to the blue of host cell cytoplasm. The larger, noninfective RBs stain blue with Giemsa stain. The Gram reaction of chlamydiae is negative or variable and is not useful in identification of the agents. Chlamydial particles and inclusions stain brightly by immunofluorescence, with group-specific, species-specific, or serovar-specific antibodies.
Fully formed, mature intracellular inclusions of C trachomatis are compact masses near the nucleus that are dark purple when stained with Giemsa stain because of the densely packed mature particles. If stained with dilute Lugol’s iodine solution, some of the inclusions of C trachomatis (but not C pneumoniae or C psittaci) appear brown because of the glycogen matrix that surrounds the particles (see Figure 27-1). In contrast, inclusions of C psittaci appear as diffuse intracytoplasmic aggregates.
Chlamydiae possess shared group (genus)–specific antigens. These are heat-stable lipopolysaccharides with 2-keto-3-deoxyoctanoic acid as an immunodominant component. Antibody to these genus-specific antigens can be detected by complement fixation (CF) and immunofluorescence. Species-specific or serovar-specific antigens are mainly outer membrane proteins. Specific antigens can best be detected by immunofluorescence, particularly using monoclonal antibodies. Specific antigens are shared by only a limited number of chlamydiae, but a given organism may contain several specific antigens. There are at least 15 serovars of C trachomatis that are separated into two biovariants that cause different clinical syndromes. The trachoma biovar includes serovars A, B, Ba, and C as well as the genital tract serovars D–K. The lymphogranuloma venereum (LGV) biovar includes serovars L1, L2, and L3. Several serovars of C psittaci can be demonstrated by CF and microimmunofluorescence (MIF) tests. Only one serovar of C pneumoniae has been described.
Chlamydiae require an intracellular habitat because of the small genome size, which make them dependent upon host cells for their development and for energy requirements. Chlamydiae grow in cultures of a variety of eukaryotic cells lines. McCoy cells treated with cycloheximide commonly are used to isolate chlamydiae; C pneumoniae grows better in HL or HEp-2 cells. All types of chlamydiae proliferate in embryonated eggs, particularly in the yolk sac.
Some chlamydiae have an endogenous metabolism similar to other bacteria. They can liberate CO2 from glucose, pyruvate, and glutamate; they also contain dehydrogenases. Nevertheless, they require energy-rich intermediates from the host cell to carry out their biosynthetic activities.
The replication of chlamydiae can be inhibited by many antibacterial drugs. Cell wall inhibitors such as penicillins and cephalosporins result in the production of morphologically defective forms but are not effective in treatment of clinical diseases. Inhibitors of protein synthesis (tetracyclines, erythromycins) are effective in most clinical infections. C trachomatis strains synthesize folates and are susceptible to inhibition by sulfonamides. Aminoglycosides are noninhibitory.
The outstanding biologic feature of infection by chlamydiae is the balance that is often reached between host and parasite, resulting in prolonged persistence of infection. Subclinical infection is the rule—and overt disease the exception—in the natural hosts of these agents. Spread from one species to another (eg, birds to humans, as in psittacosis) more frequently leads to disease. Antibodies to several antigens of chlamydiae are regularly produced by the infected host. These antibodies have little protective effect against reinfection. The infectious agent commonly persists in the presence of high antibody titers. Treatment with effective antimicrobial drugs (eg, tetracyclines) for prolonged periods may eliminate the chlamydiae from the infected host. Very early, intensive treatment may suppress antibody formation. Late treatment with antimicrobial drugs in moderate doses may suppress disease but permit persistence of the infecting agent in tissues.
The immunization of humans has been singularly unsuccessful in protecting against reinfection. Prior infection or immunization at most tends to result in milder disease upon reinfection, but at times, the accompanying hypersensitization aggravates inflammation and scarring (eg, in trachoma).
Chlamydiae are classified according to their pathogenic potential, host range, antigenic differences, and other methods. Three species that infect humans have been characterized (Table 27-1).
| Chlamydia trachomatis | Chlamydia pneumoniae | Chlamydia psittaci | |
|---|---|---|---|
| Inclusion morphology | Round, vacuolar | Round, dense | Large, variable shape, dense |
| Glycogen in inclusions | Yes | No | No |
| Elementary body morphology | Round | Pear shaped, round | Round |
| Susceptible to sulfonamides | Yes | No | No |
| Plasmid | Yes | No | Yes |
| Serovars | 15 | 1 | ≥4 |
| Natural host | Humans | Humans, animals | Birds |
| Mode of transmission | Person to person, mother to infant | Airborne person to person | Airborne bird excreta to humans |
| Major diseases | Trachoma, STDs, infant pneumonia, LGV | Pneumonia, bronchitis, pharyngitis, sinusitis | Psittacosis, pneumonia, fever of unexplained origin |
This species produces compact intracytoplasmic inclusions that contain glycogen; it is usually inhibited by sulfonamides. It includes agents of human disorders such as trachoma, inclusion conjunctivitis, nongonococcal urethritis, salpingitis, cervicitis, pneumonitis of infants, and LGV.
This species produces intracytoplasmic inclusions that lack glycogen; it is usually resistant to sulfonamides. It causes respiratory tract infections in humans.
This species produces diffuse intracytoplasmic inclusions that lack glycogen; it is usually resistant to sulfonamides. It includes agents of psittacosis in humans, ornithosis in birds, feline pneumonitis, and other animal diseases.
CHLAMYDIA TRACHOMATIS OCULAR, GENITAL, AND RESPIRATORY INFECTIONS
Humans are the natural host for C trachomatis. Monkeys and chimpanzees can be infected in the eye and genital tract. C trachomatis also replicates in cells in tissue culture. C trachomatis of different serovars replicates differently. Isolates from trachoma do not grow as well as those from LGV or genital infections. Intracytoplasmic replication results in the formation of compact inclusions with a glycogen matrix in which EBs are embedded.
TRACHOMA
Trachoma is an ancient eye disease, well described in the Ebers Papyrus, which was written in Egypt 3800 years ago. It is a chronic keratoconjunctivitis that begins with acute inflammatory changes in the conjunctiva and cornea and progresses to scarring and blindness. The C trachomatis serovars A, B, Ba, and C are associated with clinical trachoma.
The incubation period for chlamydial conjunctival infection is 3–10 days. In endemic areas, initial infection occurs in early childhood, and the onset of the long-term consequence, trachoma, is insidious. Chlamydial infection is often mixed with bacterial conjunctivitis in endemic areas, and the two together produce the clinical picture. The earliest symptoms of trachoma are lacrimation, mucopurulent discharge, conjunctival hyperemia, and follicular hypertrophy. Microscopic examination of the cornea reveals epithelial keratitis, subepithelial infiltrates, and extension of limbal vessels into the cornea (pannus). As the pannus extends downward across the cornea, there are scarring of the conjunctiva, eyelid deformities (entropion, trichiasis), and an added insult caused by eyelashes sweeping across the cornea (trichiasis). With secondary bacterial infection, loss of vision progresses over a period of years. There are, however, no systemic symptoms or signs of infection. The World Health Organization has a grading scheme for assessment of trachoma (see reference by Batteiger and Tan).
The laboratory diagnosis of chlamydial infections is also discussed in Chapter 47.
Typical cytoplasmic inclusions are found in epithelial cells of conjunctival scrapings stained with fluorescent antibody or by the Giemsa method. These occur most frequently in the early stages of the disease and on the upper tarsal conjunctiva.
Inoculation of conjunctival scrapings into cycloheximide-treated McCoy cell cultures permits growth of C trachomatis if the number of viable infectious particles is sufficiently large. Centrifugation of the inoculum into the cells increases the sensitivity of the method. The diagnosis can sometimes be made in the first passage after 2–3 days of incubation by looking for inclusions by immunofluorescence or staining with iodine or Giemsa stain.
Infected individuals often develop both group antibodies and serovar-specific antibodies in serum and in eye secretions. Immunofluorescence is the most sensitive method for their detection. Neither ocular nor serum antibodies confer significant resistance to reinfection.
Developing countries, where trachoma is endemic, generally do not have the resources to apply polymerase chain reaction (PCR) or other molecular methods to the diagnosis of C trachomatis infections of the eye. Developed countries have relatively little trachoma and little need for such tests. Thus, the molecular methods have been developed for the diagnosis of genital infections. Only research projects have used PCR in studies of trachoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree