Chapter 12 Chemotherapy of infections
• Classification of antimicrobial drugs.
• Principles of optimal antimicrobial therapy.
• Use of antimicrobial drugs: choice; combinations; chemoprophylaxis and pre-emptive suppressive therapy; chemoprophylaxis in surgery.
• Special problems with antimicrobial drugs: resistance; superinfection; masking of infections.
History
Many substances that we now know to possess therapeutic efficacy were first used in the distant past. The Ancient Greeks used male fern, and the Aztecs Chenopodium, as intestinal anthelminthics. The Ancient Hindus treated leprosy with Chaulmoogra. For hundreds of years moulds have been applied to wounds, but, despite the introduction of mercury as a treatment for syphilis (16th century), and the use of cinchona bark against malaria (17th century), the history of modern rational chemotherapy did not begin until Ehrlich1 developed the idea from his observation that aniline dyes selectively stained bacteria in tissue microscopic preparations and could selectively kill them. He invented the word ‘chemotherapy’ and in 1906 he wrote:
The antimalarials pamaquin and mepacrine were developed from dyes and in 1935 the first sulfonamide, linked with a dye (Prontosil), was introduced as a result of systematic studies by Domagk.2 The results obtained with sulfonamides in puerperal sepsis, pneumonia and meningitis were dramatic and caused a revolution in scientific and medical thinking.
In 1928, Fleming3 accidentally rediscovered the long-known ability of Penicillium fungi to suppress the growth of bacterial cultures, but put the finding aside as a curiosity. His Nobel lecture in 1945 was prophetic for our current times: ‘It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body.’
In 1939, principally as an academic exercise, Florey4 and Chain5 undertook an investigation of antibiotics, i.e. substances produced by microorganisms that are antagonistic to the growth or life of other microorganisms.6 They prepared penicillin and confirmed its remarkable lack of toxicity.7 When the preparation was administered to a policeman with combined staphylococcal and streptococcal septicaemia there was dramatic improvement; unfortunately the manufacture of penicillin in the local pathology laboratory could not keep pace with the requirements (it was also extracted from the patient’s urine and re-injected); it ran out and the patient later succumbed to infection.
the magic bullets have lost some of their magic. One solution may be to find alternatives to antibiotics when resistance appears, but there is also an urgent need for new antibiotics to be developed. Few pharmaceutical companies are now involved in antibiotic development … The high cost of development, the prolonged safety evaluation, and the probable short duration of field use and the present tendency for any new compound to induce resistance all militate against major investment in new compounds.8
(See the review by Morel and Mossialos9 on how this perverse economic incentive could be turned around.)
Classification of antimicrobial drugs
A few antimicrobials have useful activity across several of these groups. For example, metronidazole inhibits obligate anaerobic bacteria as well as some protozoa that rely on anaerobic metabolic pathways (such as Trichomonas vaginalis).
Antimicrobial drugs have also been classified broadly into:
• Bacteriostatic, i.e. those that act primarily by arresting bacterial multiplication, such as sulfonamides, tetracyclines and chloramphenicol.
• Bactericidal, i.e. those which act primarily by killing bacteria, such as penicillins, aminoglycosides and rifampicin.
The classification is in part arbitrary because most bacteriostatic drugs are bactericidal at high concentrations, under certain incubation conditions in vitro, and against some bacteria. However, there is some clinical evidence for use of conventionally bactericidal drugs for infective endocarditis and meningitis.
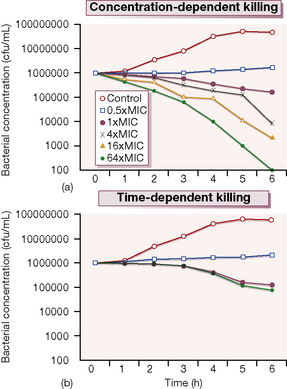
Probably more important is whether its antimicrobial effect is concentration-dependent or time-dependent. Examples of the former include the quinolones and aminoglycosides in which the outcome is related to the peak antibiotic concentration achieved at the site of infection in relation to the minimum concentration necessary to inhibit multiplication of the organism (the minimum inhibitory concentration, or MIC). These antimicrobials produce a prolonged inhibitory effect on bacterial multiplication (the post-antibiotic effect, or PAE) which suppresses growth until the next dose is given. In contrast, agents such as the β-lactams and macrolides have more modest PAEs and exhibit time-dependent killing; their concentrations should be kept above the MIC for a high proportion of the time between each dose (Fig. 12.1).
Figure 12.1 shows the results of an experiment in which a culture broth initially containing 106 bacteria per mL is exposed to various concentrations of two antibiotics, one of which exhibits concentration-dependent and the other time-dependent killing. The ‘control’ series contains no antibiotic, and the other series contain progressively higher antibiotic concentrations from 0.5 × to 64 × the MIC. Over 6 h incubation, the time-dependent antibiotic exhibits killing, but there is no difference between the 1 × MIC and 64 × MIC. The additional cidal effect of rising concentrations of the antibiotic which has concentration-dependent killing can be clearly seen.
Principles of antimicrobial chemotherapy
Make a diagnosis
It is inconsistent that the assessment of new antibiotics for therapeutic use is very much more rigorously controlled than is the introduction of diagnostic tests that direct their use – Gluud and Gluud propose a harmonised approach to the assessment and regulation of new diagnostic procedures in clinical microbiology.10
Remove barriers to cure,
e.g. drain abscesses, remove obstruction in the urinary tract and infected intravenous catheters.
Select the best drug
This involves consideration of the following factors:
• Specificity: indiscriminate use of broad-spectrum drugs promotes antimicrobial resistance and encourages opportunistic infections, e.g. with yeasts (see p. 167). At the beginning of treatment, empirical ‘best guess’ chemotherapy of reasonably broad spectrum must often be given because the susceptibility and identity of the responsible microbe is uncertain. The spectrum may be narrowed once these are microbiologically confirmed.
• Pharmacokinetic factors: to ensure that the chosen drug is capable of reaching the site of infection in adequate amounts, e.g. by crossing the blood–brain barrier.
• The patient: who may previously have exhibited allergy to a group of antimicrobials or whose routes of elimination may be impaired, e.g. by renal disease.
Prophylactic chemotherapy
for surgical and dental procedures should be of very limited duration, often only a single large dose being given (see p. 167).
Use of antimicrobial drugs
Choice
When considering ‘best guess’ therapy, infections may be categorised as those in which:
1. Choice of antimicrobial follows automatically from the clinical diagnosis because the causative organism is always the same, and is virtually always sensitive to the same drug, e.g. meningococcal septicaemia (benzylpenicillin), some haemolytic streptococcal infections, e.g. scarlet fever, erysipelas (benzylpenicillin), typhus (tetracycline), leprosy (dapsone with rifampicin).
2. The infecting organism is identified by the clinical diagnosis, but no safe assumption can be made as to its sensitivity to any one antimicrobial, e.g. tuberculosis.
3. A single infecting organism is not identified by the clinical diagnosis, e.g. in urinary tract infection or abdominal surgical wound infection.
Particularly in the second and third categories, choice of an antimicrobial may be guided by: