Chemical name
Common name
Class
Formula
Structure
Izopropylmethylphosphonofluoridate
Sarin
Nerve agent
C4H10FO2P
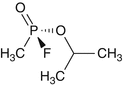
Pinacolylmethylphosphonofluoridate
Soman
Nerve agent
C7H16FO2P
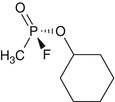
Cyclohexylmethylphosphonofluoridate
Cyclosarin
Nerve agent
C7H14FO2P
Ethyl N,N′-dimethylphosphoroamidocyanidate
Tabun
Nerve agent
C5H11N2O2P
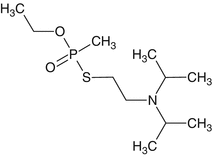
O-ethyl, S-2-diisopropylaminoethyl methylphosphonothiolate
VX
Nerve agent
C7H18NO2PS
Bis(2-chloroethyl)sulfide
Sulfur mustard
Vesicant
C4H8Cl2S
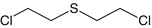
Bis(2-chloroethylthioethyl)ether
O-mustard
Vesicant
C8H16Cl2OS2
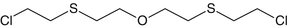
1,2-Bis(2-chloroethylthio) ethane
Sesquimustard
Vesicant
C6H12Cl2S2
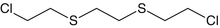
Tris-(2-chloroethyl)amine
Nitrogen mustard
Vesicant
C6H12Cl3N
2-Chlorovinyldichloroarsine
Lewisite
Vesicant
C2H2AsCl3
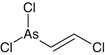
Chlorine
Chlorine
Choking agent
Cl2
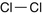
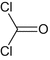
Carbonyl dichloride
Phosgene
Choking agent
CCl2O
Cyanogen chloride
CK
Blood agent
CClN
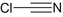
Hydrogen cyanide
Prussic acid
Blood agent
HCN
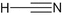
1.1.2 Nitrogen
Nitrogen, element 7 with the symbol N, is the first member of group 5A (or group 15) in the periodic table. The electronic configuration of nitrogen is: [He], 2 s2, 2p3. Nitrogen has common valences of 3 and 5. It accounts for 78 % of the atmosphere. Nitrogen molecules form an inert gas with the formula N2. Nitrogen is a component of amino acids and nucleic acids and is commonly found in a variety of foods, fertilizers and explosives. Nitrates, nitrites, amines, azides and azos are amongst prevalently existing nitrogen containing compounds (Heather 2005).
1.1.3 Sulfur
Sulfur, element 16 with the symbol S, is the second member of the group 6A (or group 16) and has the electronic configuration: [Ne], 3 s2, 3p4. Common valances of sulfur are −2, +4 and +6. The infamous smell that sulfur is commonly associated with is that of hydrogen sulfide (H2S). Sulfur, in its elemental form reacts with all metals except gold and platinum, forming sulfides. Sulfide and sulfate as well as sulfur in its elemental form are all found in nature. Sulfur compounds include sulfates, sulfites, sulfides and organosulfurs such as thiols, sulfonates and sulfonamides. The latter class comprises of synthetic compounds and posses antibacterial capabilities. In the late 1930s a newly adopted approach to combating microbial infection using Sulfanilamide, a member of the sulfonamides family of compounds lead to acute renal failure. This incident known as ‘the sulfanilamide disaster’ was caused by the solvent medium of the drug and triggered a series of studies that molded the discipline of modern toxicology (Klaassen 2007). The main commercial applications of sulfur are in the synthesis of sulfuric acid and fertilizers. Sulfur is also used in production of gunpowder, matches, insecticides and fungicides (Ede 2006).
1.2 Mustards
1.2.1 Nitrogen Mustard
Nitrogen mustard was never used in combats. However, it was initially intended to be used as a chemical warfare as its mechanism of action and symptoms are similar to those of sulfur mustard. Nitrogen mustard was soon excluded in chemical weapons production programs. Instead, nitrogen mustard compounds have been used in chemotherapy (Hanna et al. 1963; Brunton et al. 2007; Saha et al. 2013).
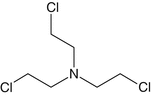
Nitrogen mustards (NMs) are colorless to pale yellow, oily liquids that evaporate slowly. Most nitrogen mustard compounds are derivatives of the following four key compounds: HN-1, HN-2, HN-3 and Iso- Pr-N(EtCl)2. Pr and Et refer to propyl and ethyl groups, respectively. HN-1 was the first of the HN series to be synthesized. HN-2 was the second compound to be developed. The synthesis of which was followed by that of HN-3. HN-2 has a fruity odor at high concentrations, and a soapy or fishy odor at low concentrations. HN-1 has a faint fishy or musty dour. It evaporates slowly and is mildly persistent (Leikin et al. 2007). Nitrogen mustard HN-3 is odorless when pure and is the most stable and most toxic of the HN series (Hoenig 2002; Hoenig 2007). Fig. 1.1 shows the structures of nitrogen mustards. Their physical properties are listed in Table 1.5.

Fig. 1.1
Nitrogen mustards
1.2.2 Sulfur Mustards
Sulfur Mustard (SM) with the chemical name bis(2-chloroethyl) sulfide or bis(β-chloroethyl) sulfide and molecular formula C4H8Cl2S (Tables 1.1 and 1.2) is a poisonous chemical agent belonging to the blister/vesicant class of compounds. Receiving its most common name from its smell, mustard is also called mustard gas, 1,1′-thiobis(2-chloroethane) and 2,2′-chlorodiethylsulfide, Lost (derived from Wilhelm Lommel and Wilhelm Steinkopf who developed a method for mass production of mustard gas for German army in 1916); HS (Hun stuff); HD (distilled sulfur mustard); Schwefel-lost; yellow cross liquid, Senfgas and Yperite which was used in Ypres in world war I (Balali-Mood et al. 2014). Although referred to as mustard gas, the compound is in liquid state at room temperature. The IUPAC name for the compound ClCH2CH2SCH2CH2Cl is 1-chloro-2-(2-chloroethylsulfanyl) ethane. It has been categorized as Group 1carcinogen to humans by the International Agency for Research on Cancer (IARC) and was listed as a toxic chemical in Schedule I by the first Annual Report on Carcinogens and Chemical Weapons Convention (1980). It is a severe irritant and vesicant of skin, eyes and lungs and was formerly used as a war gas (Balali-Mood et al. 2005; Balali‐Mood and Hefazi 2006).
Table 1.2
Examples of sulfur mustard compounds
Chemical name | Common name | Formula | Structure |
|---|---|---|---|
Bis(2-chloroethyl)sulfide | Sulfur mustard | C4H8Cl2S | 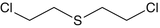 |
Bis(2-chloroethylthioethyl)ether | O-mustard | C8H16Cl2OS2 |  |
1,2-Bis(2-chloroethylthio) ethane | Sesquimustard | C6H12Cl2S2 | 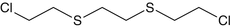 |
2-Chloroethyl chloromethyl sulfide | – | C3H6Cl2S | 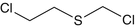 |
Bis(2-chloroethylthio)-methane | – | C5H10Cl2S2 |  |
Bis(2-chloroethylthiomethyl)-ether | – | C6H12Cl2OS2 |  |
Sulfur mustard and other members of its family of compounds are linear molecules and have similar structures. Sulfur Mustard and examples of compounds in the sulfur mustard family are shown by some examples in Table 1.2.
1.3 Applications of Mustard Compounds
As chemical weapons, chlorine and phosgene spread in the wind and therefore dilute in the air. Sulfur Mustard, on the other hand, is persistent in the environment and as such an effective battle gas. The other members of mustard compounds family – nitrogen mustards – have been used as antineoplastic drugs. Mechanism of action and symptoms of nitrogen mustards (also known as N-mustards) closely resemble those of sulfur mustard. The research studies carried out in 1946 showed that nitrogen mustard can reduce tumor growth in mice. However, several side effects have been reported in patients that were treated with nitrogen mustard. These include low blood cell counts leading to potential infections, anemia; nausea and vomiting as well as hair loss (Daniel 2011).
1.3.1 Medicinal Uses
Chemotherapy dates back to the 1940s and the use of nitrogen mustards and antifolate (folic acid antagonist) drugs (Chabner and Roberts 2005; DeVita and Chu 2008). The aim of Milton Winternitz at Yale and two other pharmacologists, Alfred Gilman and Louis Goodman was to investigate potential therapeutic effects of chemical warfare agents. Mustard was noted to destroy lymphocytes and lymphoid tissues of laboratory animals. Also, they recognized in their research that nitrogen mustards – analogs of mustard gas -could have potential chemotherapeutic effects in the treatment of lymphosarcoma. Based on their experiments sulfur mustard was less suitable for cancer treatment (Liebow and Waters 1959). In the presence of nitrogen mustard, however, the tumors shrunk and in cases, by the end of the treatment, they had disappeared. Reports on the clinical use of chlormethine (mechlorethamine) in lymphomas provided stimulus for the preparation of other drugs and resulted in development of the field of anticancer chemotherapy (Lu and Mahato 2009).
Derivatives of nitrogen mustard are used for treatment of multiple cancer diseases such as Hodgkin’s disease (cancer of the lymph nodes) and remain potent chemotherapeutic agents for malignant diseases (Ullmann and Bohnet 2003). Application of nitrogen mustards as effective anti-cancer agents is based on their ability of inducing apoptosis. Nitrogen mustard HN2 (Mechlorethamine) was found useful for chemotherapy (Gilman 1963). Analogs of HN2 have various therapeutic applications as anti-cancer drugs. Cyclophosphamide, Chlorambucil, Melphalan and Ifosfamide are also amongst nitrogen mustards with anticancer activities (Pratt 1994).
1.4 Synthesis
1.4.1 Synthesis of Sulfur Mustard
Chemists César-Mansuete Despretz and Alfred Riche both reported the synthesis of sulfur mustard from the reaction of ethylene and sulfur dichloride in 1822 and 1854, respectively (Tuorinsky 2008). Later, the reaction conditions for the synthesis of sulfur mustard were optimized by Levinstein; a dye manufacturer in England and was called the Levinstein process. Empirically, ethylene may also react with disulfur dichloride (sulfur monochloride) to produce sulfur mustard (Malhotra et al. 1999).
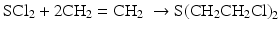 In the Levinstein process sulfur mustard is attained by the reaction of ethylene molecule with sulfur dichloride. This electrophilic addition to ethane happens in two steps and sulfur mustard forms by addition of a second molecule of alkene to the intermediate 2-chloroethylsulfenyl chloride:
In the Levinstein process sulfur mustard is attained by the reaction of ethylene molecule with sulfur dichloride. This electrophilic addition to ethane happens in two steps and sulfur mustard forms by addition of a second molecule of alkene to the intermediate 2-chloroethylsulfenyl chloride:
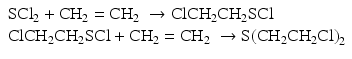 SCl2 undergoes anti addition to the olefinic bond i.e. two substituents are added to opposite sides of the double bond. In 1860, Frederick Guthrie used the above method to synthesize the compound and reported minor irritation (vesicant/blister-inducing effects) while Despeterez did not. Guthrie also reported the mustard like odor of the product (Freemantle 2014).
SCl2 undergoes anti addition to the olefinic bond i.e. two substituents are added to opposite sides of the double bond. In 1860, Frederick Guthrie used the above method to synthesize the compound and reported minor irritation (vesicant/blister-inducing effects) while Despeterez did not. Guthrie also reported the mustard like odor of the product (Freemantle 2014).
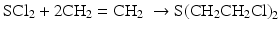
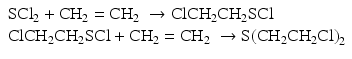
Viktor Meyer first prepared pure sulfur mustard in 1886 by the reaction of thiodiglycol with phosphorus trichloride in a two stage synthetic process (Pechura and Rall 1993; Tuorinsky 2008). Germany used the high yielding method of Meyer to manufacture mustard during World War I:
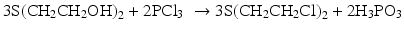 Here, the diol-compound is chlorinated with PCl3. Thiodiglycol was itself prepared by the reaction of 2-chloroethanol (ethylene chlorohydrin) with aqueous solution of potassium sulfide:
Here, the diol-compound is chlorinated with PCl3. Thiodiglycol was itself prepared by the reaction of 2-chloroethanol (ethylene chlorohydrin) with aqueous solution of potassium sulfide:
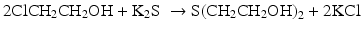 Meyer prepared 2-chloroethanol needed for the above process from the reaction of ethylene (which was synthesized by Meyer from ethanol) and carbon dioxide (CO2) with calcium hypochlorite Ca(ClO)2 known as chlorine powder or bleach powder.
Meyer prepared 2-chloroethanol needed for the above process from the reaction of ethylene (which was synthesized by Meyer from ethanol) and carbon dioxide (CO2) with calcium hypochlorite Ca(ClO)2 known as chlorine powder or bleach powder.
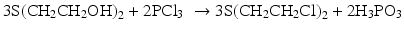
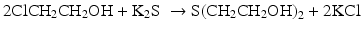
Finally in 1913, Hans Thacher Clarke replaced phosphorus trichloride (PCl3) with concentrated hydrochloric acid (HCl) in the reaction used by Victor Meyer (Puskar 2011). Steinkoff and Ludin also repeated this preparation pathway using concentrated hydrochloric acid as the chlorinating agent (Lundin and Institute 1991).
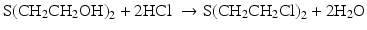 The treatment of bis(2-hydroxyethyl) sulfide with hydrogen chloride (above) was used in preparation of sulfur mustard for warfare applications.
The treatment of bis(2-hydroxyethyl) sulfide with hydrogen chloride (above) was used in preparation of sulfur mustard for warfare applications.
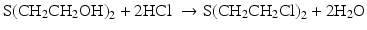
In 1945 the photochemical preparation of SM was reported through the addition of H2S to vinyl chloride:
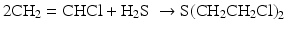 The mechanism of the photoaddition of hydrogen sulfide to olefinic double bond is a chain reaction involving free radical intermediates. The reaction occurs in the presence of peroxides (organic compounds of the type ROOR) which are initiators; they act as a source of radicals necessary to get the chain reaction started. The oxygen–oxygen bond of the peroxide is relatively weak and breaks homolytically, giving two alkoxy radicals. Heat or 2800 Å UV irradiation easily breaks the O-O bond of peroxides. Alkoxy radical abstracts a hydrogen atom from hydrogen sulfide producing HS• radical. Once HS• radical becomes available, the propagation step of the chain reaction begins. This radical is added to the alkene by a pi-bond cleavage forming a new free radical that easily separates a hydrogen atom from H2S molecule. Initiation and propagation steps of the radical addition mechanism are depicted below:
The mechanism of the photoaddition of hydrogen sulfide to olefinic double bond is a chain reaction involving free radical intermediates. The reaction occurs in the presence of peroxides (organic compounds of the type ROOR) which are initiators; they act as a source of radicals necessary to get the chain reaction started. The oxygen–oxygen bond of the peroxide is relatively weak and breaks homolytically, giving two alkoxy radicals. Heat or 2800 Å UV irradiation easily breaks the O-O bond of peroxides. Alkoxy radical abstracts a hydrogen atom from hydrogen sulfide producing HS• radical. Once HS• radical becomes available, the propagation step of the chain reaction begins. This radical is added to the alkene by a pi-bond cleavage forming a new free radical that easily separates a hydrogen atom from H2S molecule. Initiation and propagation steps of the radical addition mechanism are depicted below:
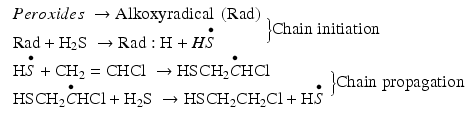 In the propagation phase, during the addition of mercaptans to double bonds, the sulfhydryl group bonds to the carbon with greater number of hydrogen atoms to give the more stable radical. In a similar way, the second hydrogen bonded to sulfur atom can be replaced and finally the sulfur mustard product is formed (Vaughan and Rust 1942).
In the propagation phase, during the addition of mercaptans to double bonds, the sulfhydryl group bonds to the carbon with greater number of hydrogen atoms to give the more stable radical. In a similar way, the second hydrogen bonded to sulfur atom can be replaced and finally the sulfur mustard product is formed (Vaughan and Rust 1942).
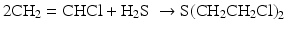
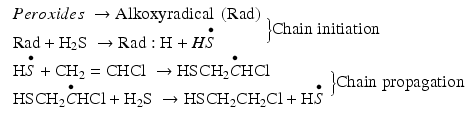
The purification of the crude compound can be achieved through three different methods namely vacuum distillation, steam distillation, and solvent extraction (Gates and Moore 1946).
1.4.1.1 Bis(2-Chloroethyl) Polysulfides
Levinstein mustard gas composes a considerable amount of bis(2-chloroethyl) polysulfides which have high sulfur content and have the general formula Sx(CH2CH2Cl)2. These polysulfides vary in composition and consist of bis(2-chloroethyl) disulfide, trisulfide, and pentasulfide. The composition of the polysulfide depends on the condition of the reaction, particularly on the temperature and the rate of addition of ethylene. The more elevated temperatures produce greater yield of SM and higher sulfur content in the polysulfide. The trisulfide compound has three sulfur atoms which are held in a linear skeleton and the pentasulfide has the similar structure with two dative bonds to the central S atom (Fig. 1.2). Disulfide preparations are of special value in the synthesis of mustard gas; and pentasulfide compound is observed in the hydrolysis of sulfur mustard. The trisulfide molecule easily takes up two additional sulfur atoms to yield the pentasulfide compound (Fuson et al. 1946).


Fig. 1.2
Structures of bis(2-chloroethyl) tri and penta sulfides
1.4.2 Synthesis of Nitrogen Mustard Compounds
Nitrogen mustards were synthesized and investigated during the 1930s. The most important nitrogen mustards were ethyl-bis(β-chloroethyl) amine (HN1), methyl-bis(β-chloroethyl) amine (HN2), tris(β-chloroethyl) amine (HN3) and isopropyl-bis(β-chloroethyl) amine (Tuorinsky 2008).
The most practical method for preparation of nitrogen mustards is from the corresponding hydroxy compounds. The ethanolamine is chlorinated with thionyl chloride (SOCl2), resulting in the synthesis of the above mentioned nitrogen mustards (Cope et al. 1946). For instance HN3 can be prepared as follows:
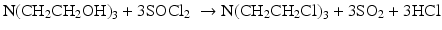 Alternatively, phosphorous trichloride can be used instead of thionyl chloride. Phosphorous trichloride, phosgene and hydrochloric acid are other alternative reagents that can replace thionyl chloride.
Alternatively, phosphorous trichloride can be used instead of thionyl chloride. Phosphorous trichloride, phosgene and hydrochloric acid are other alternative reagents that can replace thionyl chloride.
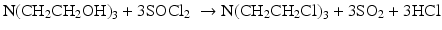
Ethylene oxide reacts with primary amines forming a mixture of mono and di-ethanolamines. Optimum conditions, as described in the equation below, can direct this reaction to yield the diethanolamine product. Preparation of N-methyl-2, 2′-dichlorodiethylamine (nitrogen mustard HN2) can be done by condensation of gaseous ethylene oxide with aqueous methylamine (25 % w/v), and treating the resultant N-methyldiethanolamine with thionyl chloride (Abrams et al. 1949). The product is then purified and separated from water, methylamine and mono- ethanolamine by distillation.
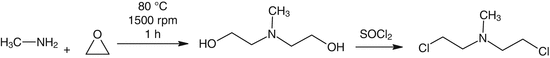

Another synthetic route is to prepare alkanolamines without employing ethylene oxide. Alkanolamine can be converted to nitrogen mustard in a chlorination step. A developed method for preparing HN1 initiates with formaldehyde and hydrogen cyanide (HCN) and follows the below steps (Et stands for ethyl group):
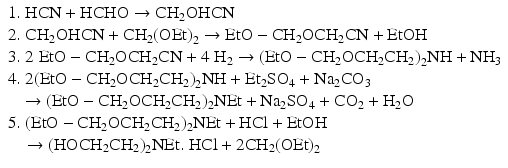 The final diethanolamine derivative is the precursor for preparation of nitrogen mustard HN1. In the above reactions, formaldehyde cyanohydrin is formed in step one. This is then followed by hydrogenation (step 3) and alkylation (steps 2 & 4). Methyl-bis(β-hydroxyethyl) amine as the precursor of HN2 can be prepared by hydrogenation of diethanolamine in the presence of formaldehyde (Cope et al. 1946).
The final diethanolamine derivative is the precursor for preparation of nitrogen mustard HN1. In the above reactions, formaldehyde cyanohydrin is formed in step one. This is then followed by hydrogenation (step 3) and alkylation (steps 2 & 4). Methyl-bis(β-hydroxyethyl) amine as the precursor of HN2 can be prepared by hydrogenation of diethanolamine in the presence of formaldehyde (Cope et al. 1946).
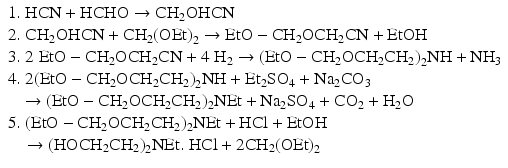
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


